Try following problems from Fogler 5e(Fogler 2016 ) .
P3-5, P3-10, P3-11, P3-12, P 4-6, P 4-8, P 4-11
We will go through some of these problems in the workshop.
P 3.12
Write the rate law for the following reactions assuming each reaction follows an elementary rate law. Give the units of k A k_A k A
C X 2 H X 6 → C X 2 H X 4 H X 2 \ce{C2H6 -> C2H4 H2} C X 2 H X 6 C X 2 H X 4 H X 2
C X 2 H X 4 + 1 2 O X 2 → C X 2 H X 4 O \ce{C2H4 + 1/2 O2 -> C2H4O} C X 2 H X 4 + 2 1 O X 2 C X 2 H X 4 O
( C H X 3 ) X 3 C O O C ( C H X 3 ) X 3 ⇌ C X 2 H X 6 + 2 C H X 3 C O C H X 3 \ce{(CH3)3COOC(CH3)3 <=> C2H6 + 2CH3COCH3} ( CH X 3 ) X 3 COOC ( CH X 3 ) X 3 C X 2 H X 6 + 2 CH X 3 COCH X 3
n C X 4 H X 10 ⇌ i C X 4 H X 10 \ce{nC4H10 <=> iC4H10} n C X 4 H X 10 i C X 4 H X 10
C H X 3 C O O C X 2 H X 5 + C X 4 H X 9 O H ⇌ C H X 3 C O O C X 4 H X 9 + C X 2 H X 5 O H \ce{ CH3COOC2H5 + C4H9OH <=> CH3COOC4H9 + C2H5OH} CH X 3 COOC X 2 H X 5 + C X 4 H X 9 OH CH X 3 COOC X 4 H X 9 + C X 2 H X 5 OH
2 C H X 3 N H X 2 ⇌ cat ( C H X 3 ) X 2 N H + N H X 3 \ce{2CH3NH2 <=>[][{cat}] (CH3)2NH + NH3} 2 CH X 3 NH X 2 cat ( CH X 3 ) X 2 NH + NH X 3
( C H X 3 C O ) X 2 O + H X 2 O ⇌ 2 C H X 3 C O O H \ce{ (CH3CO)2O + H2O <=> 2CH3COOH } ( CH X 3 CO ) X 2 O + H X 2 O 2 CH X 3 COOH
− r A = k C A -r_A = k C_A − r A = k C A
− r A = k C A C B 1 / 2 -r_A = k C_A C_B^{1/2} − r A = k C A C B 1/2
− r A = k ( C A − C B C C K ) -r_A = k \left( C_A -\frac{C_B C_C}{K} \right) − r A = k ( C A − K C B C C )
− r A = k ( C A − C B K ) -r_A = k \left( C_A -\frac{C_B}{K} \right) − r A = k ( C A − K C B )
− r A = k ( C A C B − C C C D K ) -r_A = k \left( C_A C_B -\frac{C_C C_D}{K} \right) − r A = k ( C A C B − K C C C D )
− r A ′ = k ( P A 2 − P B P C K ) -r'_A = k \left( P_A^2 -\frac{P_B P_C}{K} \right) − r A ′ = k ( P A 2 − K P B P C )
− r A = k ( C A C B − C C 2 K ) -r_A = k \left( C_A C_B -\frac{C_C^2}{K} \right) − r A = k ( C A C B − K C C 2 )
P3-10
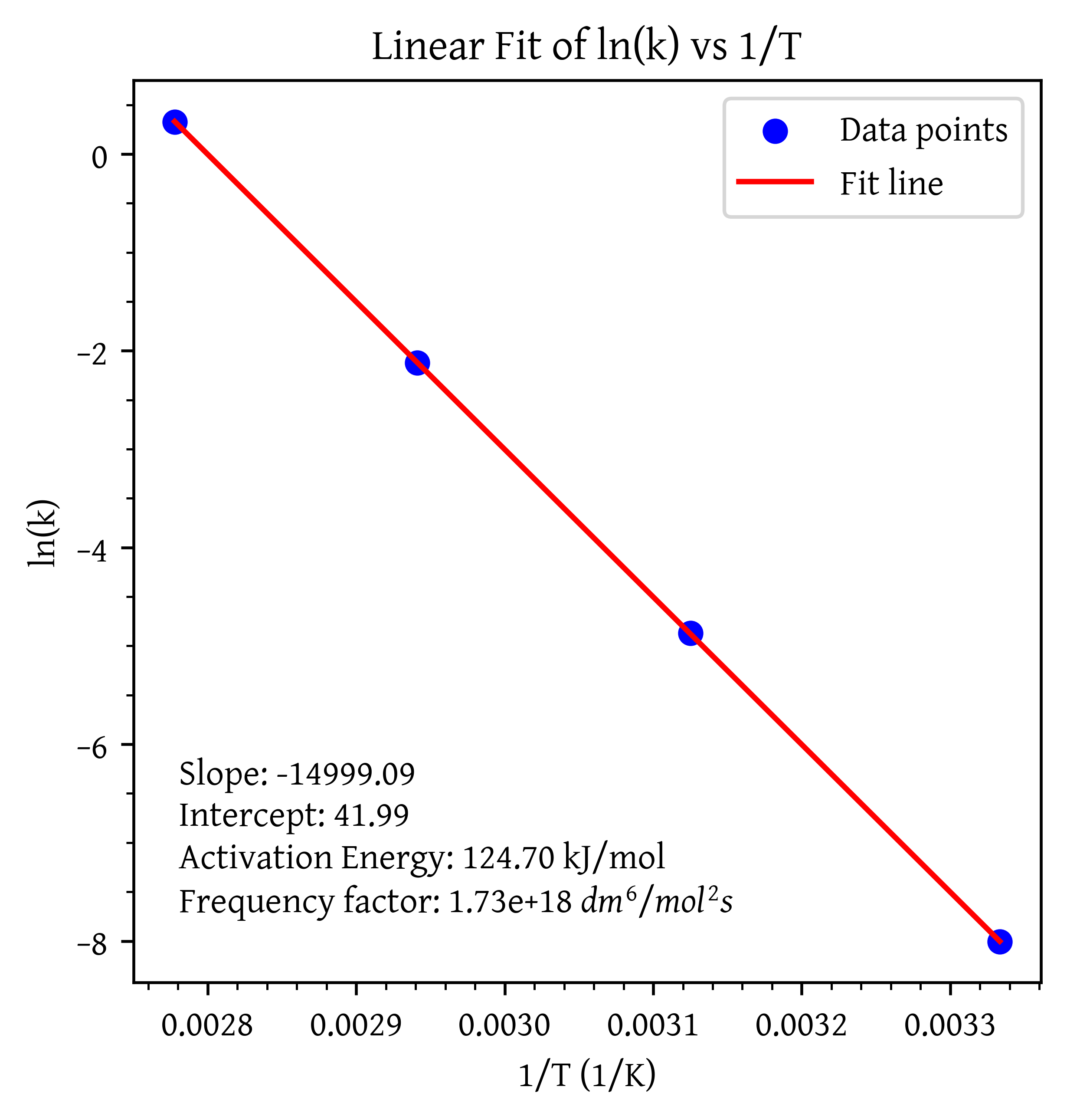
The initial reaction rate for the elementary reaction 2 A + B → 4 C \ce{2A + B -> 4C} 2 A + B 4 C
– r A ( m o l / d m 3 s ) –r_A (mol/dm^3 s) – r A ( m o l / d m 3 s ) T ( K ) T(K) T ( K )
0.002
300
0.046
320
0.72
340
8.33
360
What is the activation energy?
What is the frequency factor?
What is the rate constant as a function of temperature using Equation 1 and T 0 T_0 T 0
k ( T ) = k ( T 0 ) e x p [ E R ( 1 T 0 − 1 T ) ] (1)
k(T) = k(T_0) exp \left[ \frac{E}{R} \left( \frac{1}{T_0} - \frac{1}{T} \right)\right]
\tag{1} k ( T ) = k ( T 0 ) e x p [ R E ( T 0 1 − T 1 ) ] ( 1 )
2 A + B → 4 C \ce{2A + B -> 4C} 2 A + B 4 C
For elementary reaction
− r A = k C A 2 C B -r_A = k C_A^2 C_B − r A = k C A 2 C B
k = − r A C A 2 C B k = \frac{-r_A}{C_A^2 C_B} k = C A 2 C B − r A
At the start of reaction:
k = − r A 0 C A 0 2 C B 0 k = \frac{-r_{A0}}{C_{A0}^2 C_{B0}} k = C A 0 2 C B 0 − r A 0
import numpy as npimport matplotlib.pyplot as pltfrom scipy.stats import linregress# Data = np.array([0.002 , 0.046 , 0.72 , 8.33 ])= np.array([300 , 320 , 340 , 360 ])= 8.314 # J/mol K = 2.0 = 1.5 = r_A/ (C_A0** 2 * C_B0)= np.log(k)= 1 / T= linregress(inv_T, ln_k)= slope * inv_T + intercept= - slope* R/ 1000 # (kJ/mol) = np.exp(intercept)# Plot = 'blue' , label= 'Data points' )= 'red' , label= 'Fit line' )# Labels and title '1/T (1/K)' )'ln(k)' )'Linear Fit of ln(k) vs 1/T' )= f'Slope: { slope:.2f} ' = f'Intercept: { intercept:.2f} ' = f'Activation Energy: { activation_energy:.2f} kJ/mol' = f'Frequency factor: { A0:.2e} $dm^6/mol^2 s$' f' { slope_text} \n { intercept_text} \n { e_text} \n { a0_text} ' , xy= (0.05 , 0.25 ), xycoords= 'axes fraction' , verticalalignment= 'top' )# Show plot
k = 1.73 × 10 18 exp [ − 15000 T ]
k = 1.73 \times 10^{18}
\exp \left[
\frac{ -15000 }
{T}
\right]
k = 1.73 × 1 0 18 exp [ T − 15000 ]
P 4-8
The gas-phase reaction
1 2 N X 2 + 3 2 H X 2 → N H X 3
\ce{1/2 N2 + 3/2 H2 -> NH3}
2 1 N X 2 + 2 3 H X 2 NH X 3
is to be carried out isothermally first in a flow reactor. The molar feed is 50% H X 2 \ce{H2} H X 2 N X 2 \ce{N2} N X 2 227 ∘ C 227 \ ^{\circ}C 227 ∘ C
Construct a complete stoichiometric table.
Express the concentrations in m o l / d m 3 mol/dm^3 m o l / d m 3 C A 0 C_{A0} C A 0 δ \delta δ ϵ \epsilon ϵ H X 2 \ce{H2} H X 2
Suppose by chance the reaction is elementary with k N 2 = 40 d m 3 / m o l / s k_{N_2} = 40 \ dm^3
/mol/s k N 2 = 40 d m 3 / m o l / s
a flow reactor, and for
a constant-volume batch reactor.
Gas phase reaction
1 2 N X 2 + 3 2 H X 2 → N H X 3
\ce{1/2 N2 + 3/2 H2 -> NH3}
2 1 N X 2 + 2 3 H X 2 NH X 3
y A = 0.5 y_A = 0.5 y A = 0.5
y B = 0.5 y_B = 0.5 y B = 0.5
P = 16.4 a t m P = 16.4 atm P = 16.4 a t m
T = 227 ∘ C = 500 K T = 227 ^\circ C = 500 K T = 22 7 ∘ C = 500 K
stoichiometric table
Basis of reaction: H X 2 \ce{H2} H X 2
H X 2 + 1 3 N X 2 → 2 3 N H X 3 ; A + 1 3 B → 2 3 C
\ce{H2 + 1/3 N2 -> 2/3 NH3}; \qquad
\ce{A + 1/3 B -> 2/3 C}
H X 2 + 3 1 N X 2 3 2 NH X 3 ; A + 3 1 B 3 2 C
A
F A 0 F_{A0} F A 0 − F A 0 X -F_{A0}X − F A 0 X F A = F A 0 ( 1 − X ) F_A = F_{A0}(1 - X) F A = F A 0 ( 1 − X )
B
F B 0 = Θ B F A 0 F_{B0} = \Theta_B F_{A0} F B 0 = Θ B F A 0 − F A 0 X / 3 -F_{A0}X/3 − F A 0 X /3 F A = F A 0 ( Θ B − X / 3 ) F_A = F_{A0}(\Theta_B - X/3) F A = F A 0 ( Θ B − X /3 )
C
0
+ ( 2 / 3 ) F A 0 X + (2/3) F_{A0}X + ( 2/3 ) F A 0 X F C = ( 2 / 3 ) F A 0 X F_C = (2/3) F_{A0} X F C = ( 2/3 ) F A 0 X
_B = 1
Conc. in m o l / d m 3 mol/dm^3 m o l / d m 3 f ( X ) f(X) f ( X )
δ = c / a − b / a − 1 = 2 / 3 − 1 / 3 − 1 = − 2 / 3
\delta = c/a - b/a -1 = 2/3 - 1/3 -1 = -2/3
δ = c / a − b / a − 1 = 2/3 − 1/3 − 1 = − 2/3
ϵ = y A 0 δ = 0.5 δ = − 1 / 3
\epsilon = y_{A0} \delta = 0.5 \delta = -1/3
ϵ = y A 0 δ = 0.5 δ = − 1/3
C A 0 = 0.2
C_{A0} = 0.2
C A 0 = 0.2
C A = C A 0 ( 1 − X ) ( 1 + ϵ X ) ⋯ Constant P and T
C_A = \frac{C_{A0}(1-X)}{(1 + \epsilon X)} \cdots \text{Constant P and T}
C A = ( 1 + ϵ X ) C A 0 ( 1 − X ) ⋯ Constant P and T
C A = C A 0 ( 1 − X ) ( 1 + X / 3 ) → X = 0.6 → C A = 0.2 ( 1 − 0.6 ) ( 1 + 0.6 / 3 ) = 0.1 m o l / d m 3
C_A = \frac{C_{A0}(1-X)}{(1 + X/3)}
\rightarrow X = 0.6 \rightarrow
C_A = \frac{0.2 (1 - 0.6)}{(1 + 0.6/3)} = 0.1 mol/dm^3
C A = ( 1 + X /3 ) C A 0 ( 1 − X ) → X = 0.6 → C A = ( 1 + 0.6/3 ) 0.2 ( 1 − 0.6 ) = 0.1 m o l / d m 3
C C = 2 3 C A 0 ( X ) ( 1 + ϵ X ) → X = 0.6 → C C = 2 3 0.2 × 0.6 ( 1 + 0.6 / 3 ) = 0.1 m o l / d m 3
C_C = \frac{2}{3} \frac{C_{A0}(X)}{(1 + \epsilon X)}
\rightarrow X = 0.6 \rightarrow
C_C = \frac{2}{3} \frac{0.2 \times 0.6}{(1 + 0.6/3)} = 0.1 mol/dm^3
C C = 3 2 ( 1 + ϵ X ) C A 0 ( X ) → X = 0.6 → C C = 3 2 ( 1 + 0.6/3 ) 0.2 × 0.6 = 0.1 m o l / d m 3
− r A -r_A − r A f ( X ) f(X) f ( X ) k N 2 = 40 d m 3 / m o l s k_{N_2} = 40 dm^3/mol s k N 2 = 40 d m 3 / m o l s
Flow reactor
− r N 2 = k N 2 C N 2 1 / 2 C H 2 3 / 2
-r_{N_2} = k_{N_2} C_{N_2}^{1/2} C_{H_2}^{3/2}
− r N 2 = k N 2 C N 2 1/2 C H 2 3/2
− r N 2 = 40 [ C A 0 ( Θ B − X / 3 ) ( 1 + ϵ X ) ] 1 / 2 [ C A 0 ( 1 − X ) ( 1 + ϵ X ) ] 3 / 2
-r_{N_2} = 40 \left[ \frac{C_{A0} (\Theta_B - X/3)}{(1 + \epsilon X)} \right]^{1/2}
\left[ \frac{C_{A0} (1 - X)}{(1 + \epsilon X)} \right]^{3/2}
− r N 2 = 40 [ ( 1 + ϵ X ) C A 0 ( Θ B − X /3 ) ] 1/2 [ ( 1 + ϵ X ) C A 0 ( 1 − X ) ] 3/2
− r N 2 = 40 C A 0 2 [ ( 1 − X ) ( 1 − X / 3 ) ] 3 / 2 = 1.6 [ ( 1 − X ) ( 1 − X / 3 ) ] 3 / 2
-r_{N_2} = 40 C_{A0}^2 \left[ \frac{(1- X)}{(1 - X/3)} \right]^{3/2}
= 1.6 \left[ \frac{(1- X)}{(1 - X/3)} \right]^{3/2}
− r N 2 = 40 C A 0 2 [ ( 1 − X /3 ) ( 1 − X ) ] 3/2 = 1.6 [ ( 1 − X /3 ) ( 1 − X ) ] 3/2
For constant volume batch reactor
− r N 2 = k N 2 C N 2 1 / 2 C H 2 3 / 2
-r_{N_2} = k_{N_2} C_{N_2}^{1/2} C_{H_2}^{3/2}
− r N 2 = k N 2 C N 2 1/2 C H 2 3/2
− r N 2 = 40 C A 0 2 ( 1 − X / 3 ) 1 / 2 ( 1 − X ) 3 / 2 = 1.6 ( 1 − X / 3 ) 1 / 2 ( 1 − X ) 3 / 2
-r_{N_2} = 40 C_{A0}^2 (1- X/3)^{1/2} (1 - X)^{3/2}
= 1.6 (1- X/3)^{1/2} (1 - X)^{3/2}
− r N 2 = 40 C A 0 2 ( 1 − X /3 ) 1/2 ( 1 − X ) 3/2 = 1.6 ( 1 − X /3 ) 1/2 ( 1 − X ) 3/2
P 4-11
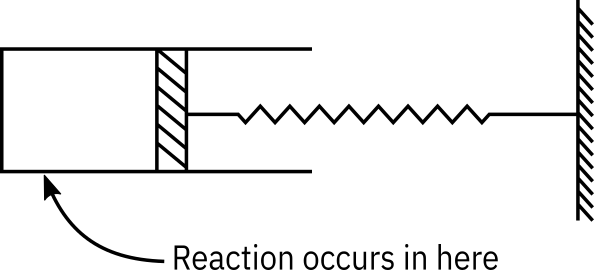
Consider a cylindrical batch reactor that has one end fitted with a frictionless piston attached to a spring (See Figure Figure 1 ). The reaction
A + B → 8 C
\ce{A + B -> 8 C}
A + B 8 C
with the rate law
− r A = k 1 C A 2 C B -r_A = k_1 C_A^2 C_B − r A = k 1 C A 2 C B
is taking place in this type of reactor.
Write the rate law solely as a function of conversion, numerically evaluating all possible symbols.
What is the conversion and rate of reaction when V = 0.2 f t 3 V=0.2 \ ft^3 V = 0.2 f t 3
Additional information:
Equal moles of A and B are present at t 0 t_0 t 0
Initial volume: 0.15 f t 3 0.15 \ ft^3 0.15 f t 3
Value of k 1 : 1.0 ( f t 3 / l b m o l ) 2 ⋅ s − 1 k_1 : 1.0 \ (ft^3 /lb mol)^2 \cdot s^{-1} k 1 : 1.0 ( f t 3 / l bm o l ) 2 ⋅ s − 1
The spring constant is such that the relationship between the volume of the reactor and pressure within the reactor is
V = ( 0.1 ) ( P ) V = (0.1)\ (P) V = ( 0.1 ) ( P ) f t 3 ft^3 f t 3 a t m atm a t m
Temperature of system (considered constant): 140 ∘ F 140 \ ^{\circ}F 140 ∘ F
Gas constant: 0.73 f t 3 a t m / l b m o l ⋅ ∘ R 0.73 \ ft^3 atm/lb mol \cdot ^{\circ}R 0.73 f t 3 a t m / l bm o l ⋅ ∘ R
Rate law as f(X)
y A 0 = N A 0 N A 0 + N B 0 = 0.5
y_{A0} = \frac{N_{A0}}{N_{A0} + N_{B0}} = 0.5
y A 0 = N A 0 + N B 0 N A 0 = 0.5
δ = 8 − 1 − 1 = 6 \delta = 8 - 1 - 1 = 6 δ = 8 − 1 − 1 = 6
ϵ = y A 0 δ = 3 \epsilon = y_{A0} \delta = 3 ϵ = y A 0 δ = 3
V = V 0 ( 1 + ϵ X ) P 0 P T T 0
V = V_0 (1 + \epsilon X) \frac{P_0}{P} \frac{T}{T_0}
V = V 0 ( 1 + ϵ X ) P P 0 T 0 T
P 0 = 10 V 0 ; P = 10 V P_0 = 10 V_0; P = 10 V P 0 = 10 V 0 ; P = 10 V
V = 10 V 0 2 10 V ( 1 + ϵ X )
V = \frac{10 V_0^2}{10 V} (1 + \epsilon X)
V = 10 V 10 V 0 2 ( 1 + ϵ X )
V 2 = V 0 2 ( 1 + ϵ X )
V^2 = V_0^2 (1 + \epsilon X)
V 2 = V 0 2 ( 1 + ϵ X )
N A = N A 0 ( 1 − X ) ; N B = N A 0 ( Θ B − X )
N_A = N_{A0}(1 - X); N_B = N_{A0} (\Theta_B - X)
N A = N A 0 ( 1 − X ) ; N B = N A 0 ( Θ B − X )
Θ B = N B 0 N A 0 = 1 \Theta_B = \frac{N_{B0}}{N_{A0}} = 1 Θ B = N A 0 N B 0 = 1
− r A = k C A 2 C B = k N A 2 N B V 2 = k N A 0 3 ( 1 − X ) 3 V 0 3 ( 1 + ϵ X ) 3 / 2
-r_A = k C_A^2 C_B = \frac{k N_A^2 N_B}{V^2}
= \frac{k N_{A0}^3 (1-X)^3}{V_0^3 (1 + \epsilon X)^3/2}
− r A = k C A 2 C B = V 2 k N A 2 N B = V 0 3 ( 1 + ϵ X ) 3 /2 k N A 0 3 ( 1 − X ) 3
N A 0 V 0 = y A 0 P R T
\frac{N_{A0}}{V_0} = \frac{y_{A0} P}{RT}
V 0 N A 0 = RT y A 0 P
∴ − r A = k ( y A 0 P 0 R T ) 3 ( ( 1 − X ) ( 1 + ϵ X ) 1 / 2 ) 3
\therefore -r_A = k \left( \frac{y_{A0} P_0}{RT} \right)^3
\left( \frac{(1-X)}{(1 + \epsilon X)^{1/2}} \right)^3
∴ − r A = k ( RT y A 0 P 0 ) 3 ( ( 1 + ϵ X ) 1/2 ( 1 − X ) ) 3
− r A = 5.02 × 10 − 9 ( ( 1 − X ) ( 1 + ϵ X ) 1 / 2 ) 3
-r_A = 5.02 \times 10^{-9} \left( \frac{(1-X)}{(1 + \epsilon X)^{1/2}} \right)^3
− r A = 5.02 × 1 0 − 9 ( ( 1 + ϵ X ) 1/2 ( 1 − X ) ) 3
X X X − r A -r_A − r A V = 0.2 f t 3 V = 0.2 ft^3 V = 0.2 f t 3
V 2 = V 0 2 ( 1 + ϵ X )
V^2 = V_0^2 (1 + \epsilon X)
V 2 = V 0 2 ( 1 + ϵ X )
( 0.2 ) 2 = ( 0.15 ) 2 ( 1 + 3 X ) ⇒ X = 0.259
(0.2)^2 = (0.15)^2 (1 + 3 X) \Rightarrow X = 0.259
( 0.2 ) 2 = ( 0.15 ) 2 ( 1 + 3 X ) ⇒ X = 0.259
− r A = 8.623 × 10 − 10 l b m o l / f t 3 s
-r_A = 8.623 \times 10^{-10} lbmol/ft^3 s
− r A = 8.623 × 1 0 − 10 l bm o l / f t 3 s
References
Fogler, H. Scott. 2016. Elements of Chemical Reaction Engineering . Fifth edition. Boston: Prentice Hall.
Citation BibTeX citation:
@online{utikar2024,
author = {Utikar, Ranjeet},
title = {Solutions to Workshop 03: {Rate} Law and Stoichiometry},
date = {2024-03-10},
url = {https://cre.smilelab.dev/content/workshops/03-rate-law-and-stoichiometry/solutions.html},
langid = {en}
}
For attribution, please cite this work as:
Utikar, Ranjeet. 2024.
“Solutions to Workshop 03: Rate Law and
Stoichiometry.” March 10, 2024.
https://cre.smilelab.dev/content/workshops/03-rate-law-and-stoichiometry/solutions.html .