Workshop 04: Isothermal reactor design
Lecture notes for chemical reaction engineering
Solutions to these problems are uploaded at Workshop 4 solutions
Try following problems from Fogler 5e (Fogler 2016).
P 5-7, P 5-8, P 5-9, P 5-11, P 5-24, P 6-4, P 6-6, P 6-7
We will go through some of these problems in the workshop.
P 5-7
The gas-phase reaction
follows an elementary rate law and is to be carried out first in a PFR and then in a separate experiment in a CSTR. When pure A is fed to a 10 dm3 PFR at 300 K and a volumetric flow rate of 5 dm3/s, the conversion is 80%. When a mixture of 50% A and 50% inert (I) is fed to a 10 dm3 CSTR at 320 K and a volumetric flow rate of 5 dm3/s, the conversion is also 80%. What is the activation energy in cal/mol?
P 5-8
The elementary gas-phase reaction
takes place isobarically and isothermally in a PFR where 63.2% conversion is achieved. The feed is pure A.
It is proposed to put a CSTR of equal volume upstream of the PFR. Based on the entering molar flow rate to A to the first reactor, what will be the intermediate from the CSTR, X1 , and exit conversion from the PFR, X2 , based on the feed to first reactor?
The entering flow rates and all other variables remain the same as that for the single PFR.
P 5-9
The liquid-phase reaction
follows an elementary rate law and is carried out isothermally in a flow system. The concentrations of the A and B feed streams are 2 M before mixing. The volumetric flow rate of each stream is 5 dm3/min, and the entering temperature is 300 K. The streams are mixed immediately before entering. Two reactors are available. One is a gray, 200.0 dm3 CSTR that can be heated to 77 C or cooled to 0 C, and the other is a white, 800.0 dm3 PFR operated at 300 K that cannot be heated or cooled but can be painted red or black. Note that at 300 K and E = 20 kcal/mol.
Which reactor and what conditions do you recommend? Explain the reason for your choice (e.g., color, cost, space available, weather conditions). Back up your reasoning with the appropriate calculations.
How long would it take to achieve 90% conversion in a 200 dm3 batch reactor with CA0 = CB0 = 1 M after mixing at a temperature of 77C?
What would your answer to part (b) be if the reactor were cooled to 0C?
What conversion would be obtained if the CSTR and PFR were operated at 300 K and connected in series? In parallel with 5 mol/min to each?
Keeping Table 1 in mind, what batch reactor volume would be necessary to process the same amount of species A per day as the flow reactors, while achieving 90% conversion?
P 5-11
The irreversible elementary gas-phase reaction
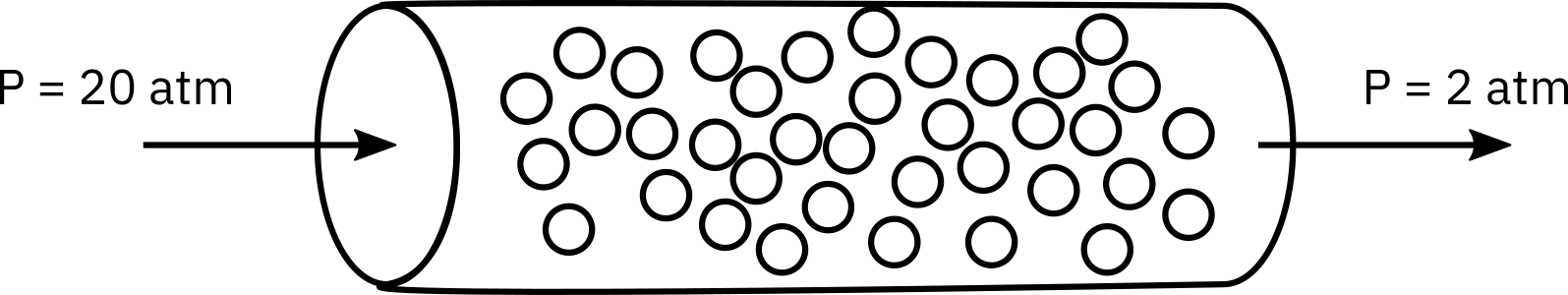
is carried out isothermally at 305 K in a packed-bed reactor with 100 kg of catalyst.
The entering pressure was 20 atm and the exit pressure is 2 atm. The feed is equal molar in A and B and the flow is in the turbulent flow regime, with FA0 = 10 mol/min and CA0 = 0.4 mol/dm3. Currently 80% conversion is achieved. What would be the conversion if the catalyst particle size were doubled and everything else remained the same?
P 5-24
The gas-phase reaction
takes place isothermally at 300 K in a packed-bed reactor in which the feed is equal molar in A and B with CA0 = 0.1 mol/dm3. The reaction is second order in A and zero order in B. Currently, 50% conversion is achieved in a reactor with 100 kg of catalysts for a volumetric flow rate 100 dm3/min. The pressure-drop parameter, , is = 0.0099 kg–1. If the activation energy is 10,000 cal/mol, what is the specific reaction rate constant at 400 K?
P 6-4
The elementary gas-phase reaction
is carried out isothermally at 400 K in a flow reactor with no pressure drop. The specific reaction rate at 50C is 10-4 min-1 (from pericosity data) and the activation energy is 85 kJ/mol. Pure di-tert-butyl peroxide enters the reactor at 10 atm and 127C and a molar flow rate of 2.5 mol/min, i.e., FA = 2.5 mol/min.
Use the algorithm for molar flow rates to formulate and solve the problem. Plot FA, FB, FC, and then X as a function of plug-flow reactor volume and space time to achieve 90% conversion.
Calculate the plug-flow volume and space time for a CSTR for 90% conversion.
P 6-6
(Membrane reactor) The first-order, gas-phase, reversible reaction
is taking place in a membrane reactor. Pure A enters the reactor, and B diffuses out through the membrane. Unfortunately, a small amount of the reactant A also diffuses through the membrane.
Plot and analyze the flow rates of A, B, and C and the conversion X down the reactor, as well as the flow rates of A and B through the membrane.
Next, compare the conversion profiles in a conventional PFR with those of a membrane reactor from part (a). What generalizations can you make?
Would the conversion of A be greater or smaller if C were diffusing out instead of B?
Discuss qualitatively how your curves would change if the temperature were increased significantly or decreased significantly for an exothermic reaction. Repeat the discussion for an endothermic reaction.
Additional information:
| k = 10 min-1 | FA0 = 100 mol/min |
| KC = 0.01 mol/dm3 | = 100 dm3/min |
| kCA= 1 min-1 | Vreactor = 20 dm3 |
| kCB = 40 min-1 |
P 6-7
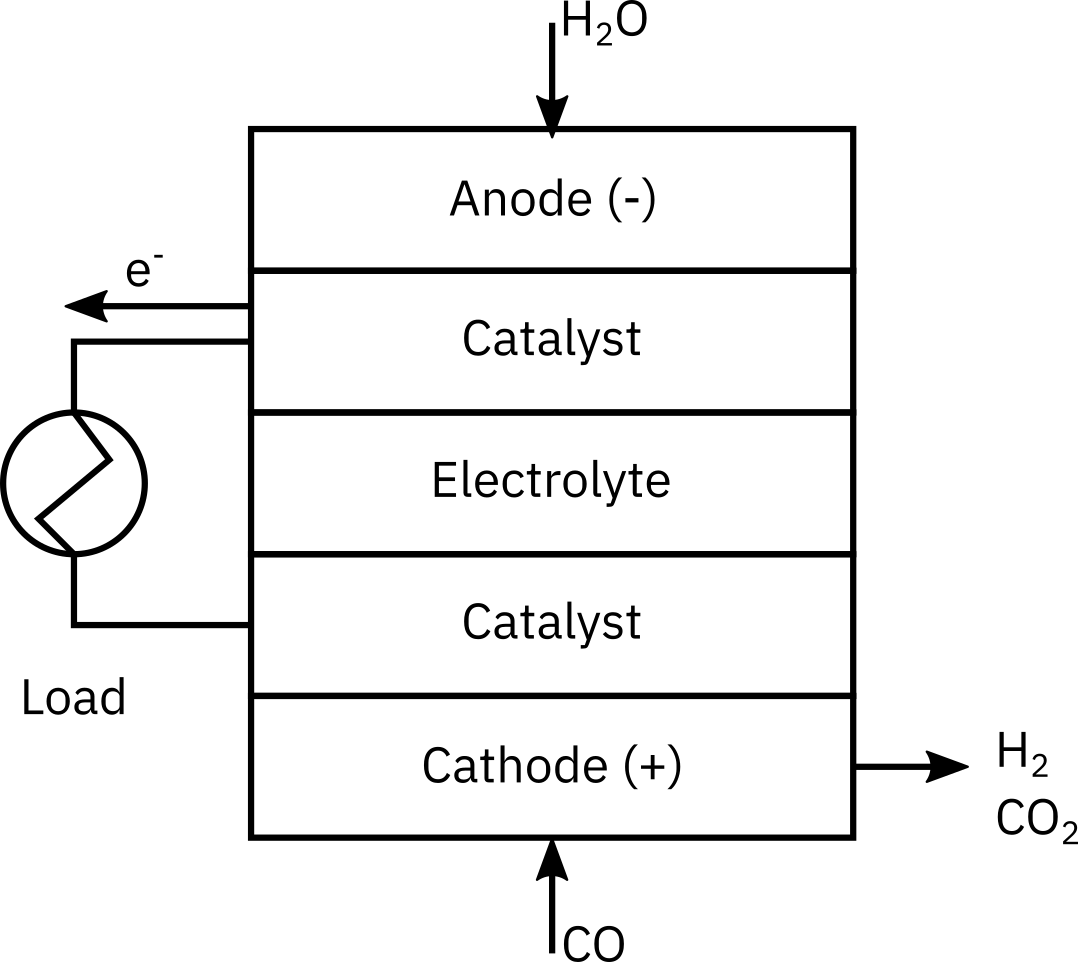
Fuel Cells Rationale. With the focus on alternative clean-energy sources, we are moving toward an increased use of fuel cells to operate appliances ranging from computers to automobiles. For example, the hydrogen/oxygen fuel cell produces clean energy as the products are water and electricity, which may lead to a hydrogen-based economy instead of a petroleum-based economy. A large component in the processing train for fuel cells is the water-gas shift membrane reactor. (M. Gummala, N. Gupla, B. Olsomer, and Z. Dardas, Paper 103c, 2003, AIChE National Meeting, New Orleans, LA.)
Here, CO and water are fed to the membrane reactor containing the catalyst. Hydrogen can diffuse out the sides of the membrane, while , , and cannot. Based on the following information, plot the concentrations and molar flow rates of each of the reacting species down the length of the membrane reactor.
Assume the following: The volumetric feed is 10 dm3/min at 10 atm, and the equimolar feed of CO and water vapor with CT0 = 0.4 mol/dm3. The equilibrium constant is Ke = 1.44, with k = 1.37 , and the mass transfer coefficient = 0.1
(Hint: First calculate the entering molar flow rate of CO and then relate FA and X.)
What is the membrane reactor volume necessary to achieve 85% conversion of CO?
Sophia wants you to compare the MR with a conventional PFR. What will you tell her?
For that same membrane reactor volume, Nicolas wants to know what would be the conversion of CO if the feed rate were doubled?
References
Citation
@online{utikar2024,
author = {Utikar, Ranjeet},
title = {Workshop 04: {Isothermal} Reactor Design},
date = {2024-03-19},
url = {https://cre.smilelab.dev/content/workshops/04-isothermal-reactor-design/},
langid = {en}
}