Workshop 02: Conversion and reactor sizing
Lecture notes for chemical reaction engineering
Try following problems from Fogler 5e(Fogler 2016).
P2-3, P2-4, P2-7, P2-10.
We will go through some of these problems in the workshop.
A collab notebook that contains specimen code can be obtained by clicking on link below.
Solutions to these problems are uploaded at Workshop 2 solutions
- P2-3: You have two CSTRs and two PFRs, each with a volume of . Use Figure 1 to calculate the conversion for each of the reactors in the following arrangements.
Two CSTRs in series.
Two PFRs in series.
Two CSTRs in parallel with the feed, , divided equally between the two reactors.
Two PFRs in parallel with the feed divided equally between the two reactors.
A CSTR and a PFR in parallel with the flow equally divided. Calculate the overall conversion,
with
and
A PFR followed by a CSTR.
A CSTR followed by a PFR.
A PFR followed by two CSTRs. Is this arrangement a good arrangement or is there a better one?
The data from Figure 1 is provided in file workshop-02-problem-1-data.csv
P2-4: The exothermic reaction of stillbene (A) to form the economically important trospophene (B) and methane (C), i.e.,
was carried out adiabatically and the following data recorded:
The entering molar flow rate of A was .
- What are the PFR and CSTR volumes necessary to achieve 40% conversion?
- Over what range of conversions would the CSTR and PFR reactor volumes be identical?
- What is the maximum conversion that can be achieved in a CSTR?
- What conversion can be achieved if a PFR is followed in series by a CSTR?
- What conversion can be achieved if a CSTR is followed in a series by a PFR?
- Plot the conversion and rate of reaction as a function of PFR reactor volume up to a volume of .
The data from Table 1 is provided in file workshop-02-problem-2.csv
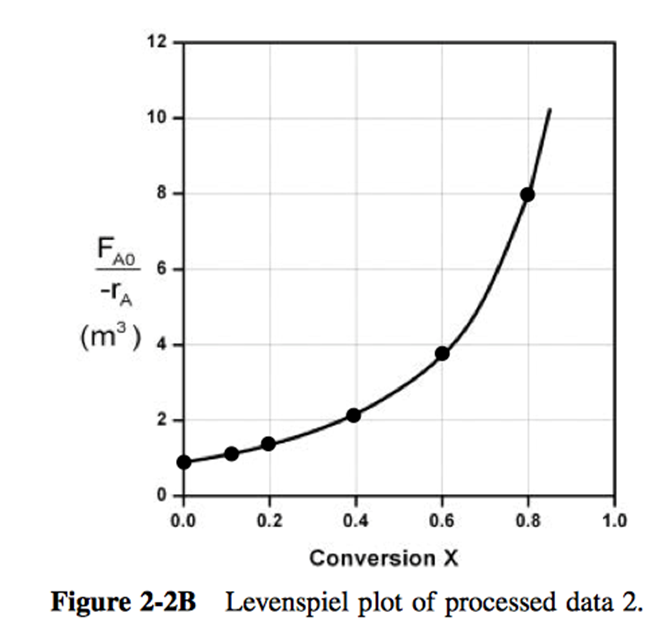
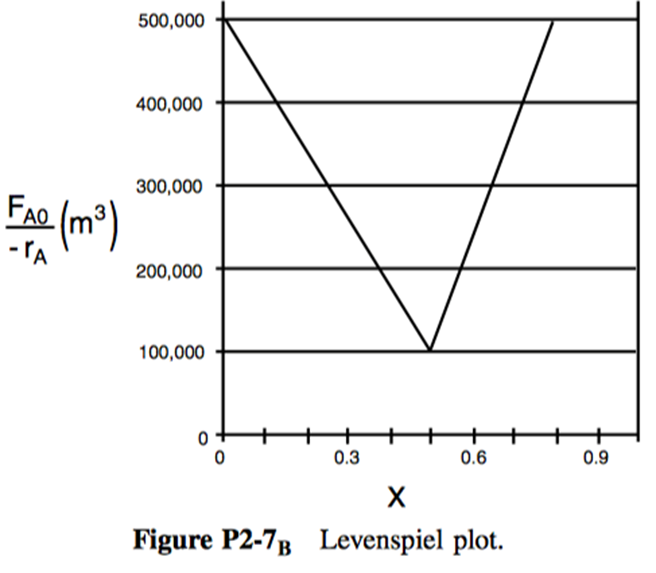
P2-7: The adiabatic exothermic irreversible gas-phase reaction
is to be carried out in a flow reactor for an equimolar feed of A and B. A Levenspiel plot for this reaction is shown in Figure 2 .
- What PFR volume is necessary to achieve 50% conversion?
- What CSTR volume is necessary to achieve 50% conversion?
- What is the volume of a second CSTR added in series to the first CSTR (Part b) necessary to achieve an overall conversion of 80%?
- What PFR volume must be added to the first CSTR (Part b) to raise the conversion to 80%?
- What conversion can be achieved in a CSTR? In a PFR?
- Think critically to critique the answers (numbers) to this problem.
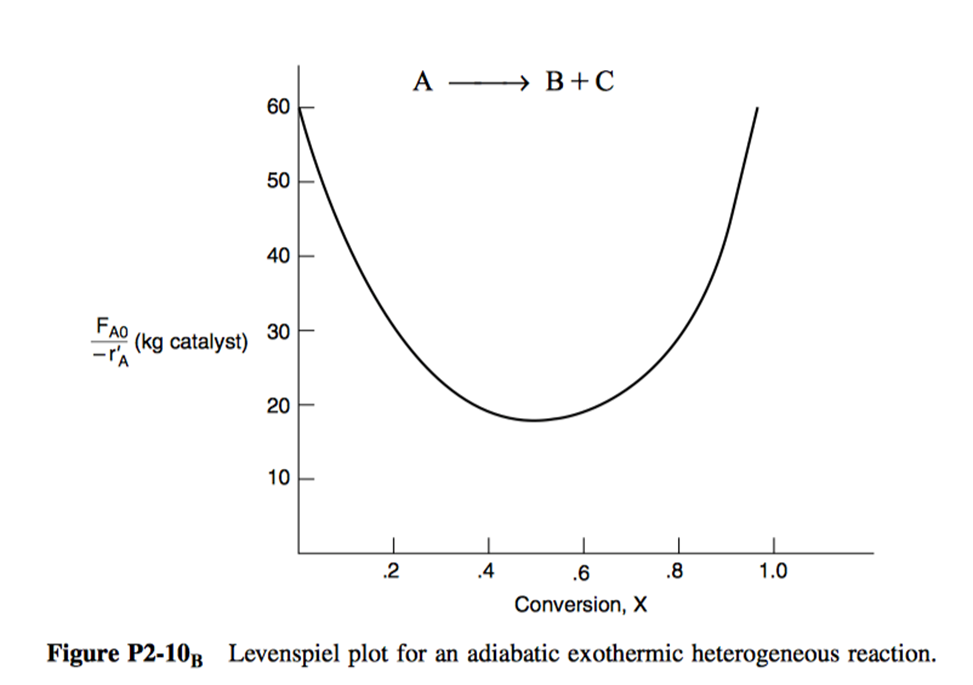
P2.10: The curve shown in Figure 3 is typical of a gas-solid catalytic exothermic reaction carried out adiabatically.
- Assuming that you have a fluidized CSTR and a PBR containing equal weights of catalyst, how should they be arranged for this adiabatic reaction? Use the smallest amount of catalyst weight to achieve 80% conversion of A.
- What is the catalyst weight necessary to achieve 80% conversion in a fluidized CSTR?
- What fluidized CSTR weight is necessary to achieve 40% conversion?
- What PBR weight is necessary to achieve 80% conversion?
- What PBR weight is necessary to achieve 40% conversion?
- Plot the rate of reaction and conversion as a function of PBR catalyst weight, W.
Additional information: FA0 = 2 mol/s.
The data from Figure 3 is provided in file workshop-02-problem-4.csv
References
Citation
@online{utikar2024,
author = {Utikar, Ranjeet},
title = {Workshop 02: {Conversion} and Reactor Sizing},
date = {2024-02-28},
url = {https://cre.smilelab.dev/content/workshops/02-conversion-and-reactor-sizing/},
langid = {en}
}