Workshop 05: Collection and analysis of rate data
Lecture notes for chemical reaction engineering
Solutions to these problems are uploaded at Workshop 5 solutions
Try following problems from Fogler 5e (Fogler 2016). P 7-4, P 7-5, P 7-6, P 7-7, P 7-10
We will go through some of these problems in the workshop.
P 7-4
When arterial blood enters a tissue capillary, it exchanges oxygen and carbon dioxide with its environment, as shown in this diagram.
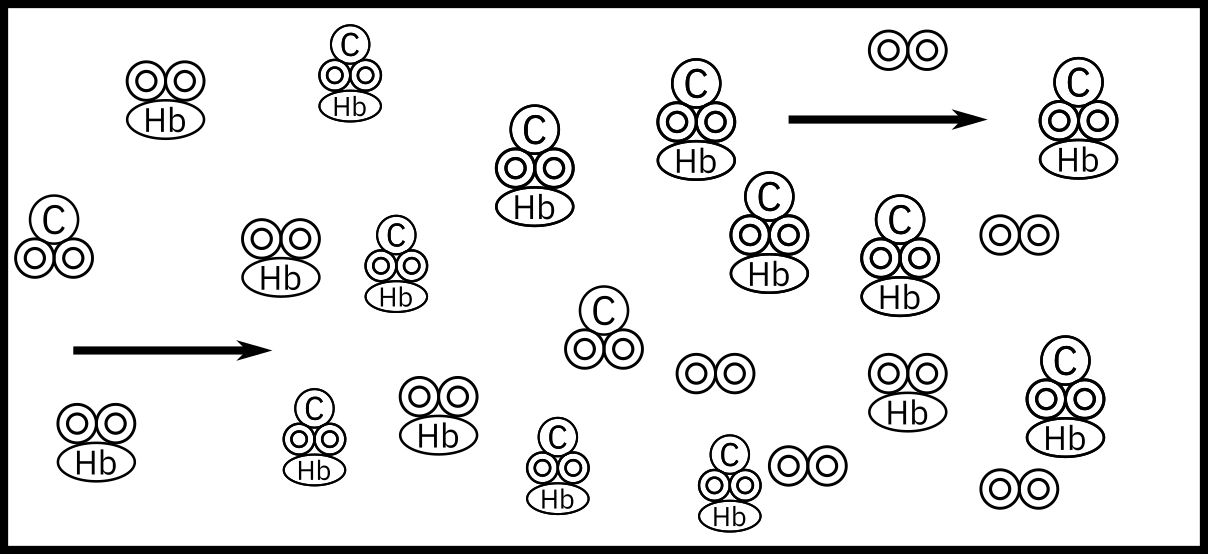
The kinetics of this deoxygenation of hemoglobin in blood was studied with the aid of a tubular reactor by Nakamura and Staub (J. Physiol., 173, 161).
Although this is a reversible reaction, measurements were made in the initial phases of the decomposition so that the reverse reaction could be neglected. Consider a system similar to the one used by Nakamura and Staub: the solution enters a tubular reactor (0.158 cm in diameter) that has oxygen electrodes placed at 5-cm intervals down the tube. The solution flow rate into the reactor is 19.6 cm3/s with CA0 = 2.33 × 10–6 mol/cm3.
| Electrode position | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Percent decomposition of | 0.00 | 1.93 | 3.82 | 5.68 | 7.48 | 9.25 | 11.00 |
Using the method of differential analysis of rate data, determine the reaction order and the forward specific reaction-rate constant k1 for the deoxygenation of hemoglobin.
Repeat using regression.
P 7-5
The liquid-phase irreversible reaction
is carried out in a CSTR. To learn the rate law, the volumetric flow rate, , (hence ) is varied and the effluent concentrations of species A are recorded as a function of the space time t. Pure A enters the reactor at a concentration of 2 mol/ dm3. Steady-state conditions exist when the measurements are recorded.
| Run | 1 | 2 | 3 | 4 | 5 |
| (min) | 15 | 38 | 100 | 300 | 1200 |
| CA (mol/dm3) | 1.5 | 1.25 | 1.0 | 0.75 | 0.5 |
Determine the reaction order and specific reaction-rate constant.
If you were to repeat this experiment to determine the kinetics, what would you do differently? Would you run at a higher, lower, or the same temperature? If you were to take more data, where would you place the measurements (e.g., )?
It is believed that the technician may have made a dilution factor-of-10 error in one of the concentration measurements. What do you think? How do your answers compare using regression (Polymath or other software) with those obtained by graphical methods?
Note: All measurements were taken at steady-state conditions.
P 7-6
The reaction
was carried out in a constant-volume batch reactor where the following concentration measurements were recorded as a function of time.
| t (min) | 0 | 5 | 9 | 15 | 22 | 30 | 40 | 60 |
| CA (mol/dm3) | 2 | 1.6 | 1.35 | 1.1 | 0.87 | 0.70 | 0.53 | 0.35 |
Use nonlinear least squares (i.e., regression) and one other method to determine the reaction order, , and the specific reaction rate, k.
Nicolas Bellini wants to know, if you were to take more data, where would you place the points? Why?
Prof. Dr. Sven Köttlov from Jofostan University always asks his students, if you were to repeat this experiment to determine the kinetics, what would you do differently? Would you run at a higher, lower, or the same temperature? Take different data points? Explain.
It is believed that the technician made a dilution error in the concentration measured at 60 min.
What do you think? How do your answers compare using regression (Polymath or other software) with those obtained by graphical methods?
P 7-7
The following data were reported [from C. N. Hinshelwood and P. J. Ackey, Proc. R. Soc. (Lond)., A115, 215] for a gas-phase constant-volume decomposition of dimethyl ether at 504C in a batch reactor. Initially, only was present.
| Time (s) | 390 | 777 | 1195 | 3155 | |
| Total Pressure (mmHg) | 408 | 488 | 562 | 799 | 931 |
Why do you think the total pressure measurement at t = 0 is missing? Can you estimate it?
Assuming that the reaction
is irreversible and goes virtually to completion, determine the reaction order and specific reaction rate k.
What experimental conditions would you suggest if you were to obtain more data?
How would the data and your answers change if the reaction were run at a higher temperature? A lower temperature?
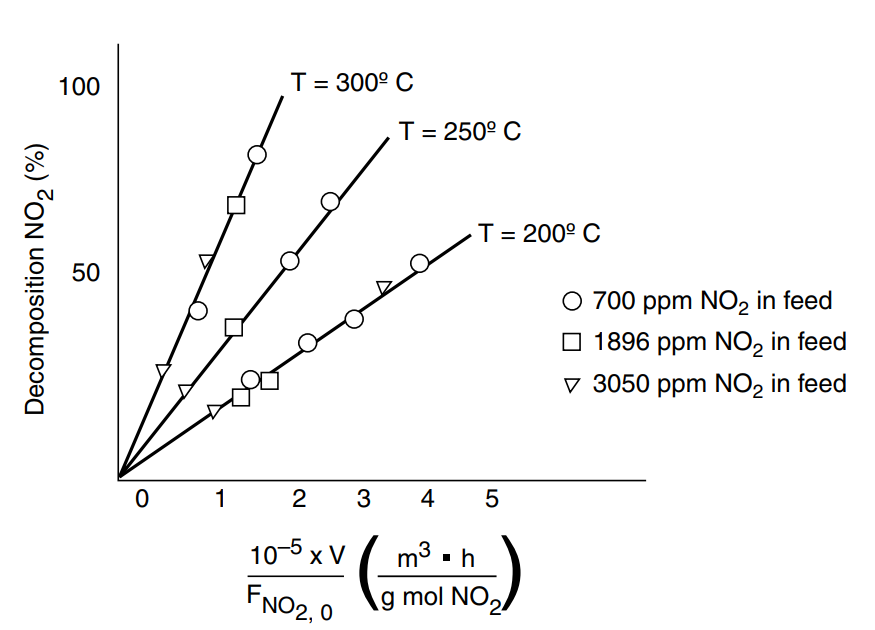
P 7-10
Tests were run on a small experimental reactor used for decomposing nitrogen oxides in an automobile exhaust stream. In one series of tests, a nitrogen stream containing various concentrations of was fed to a reactor, and the kinetic data obtained are shown in Figure P7-10. Each point represents one complete run. The reactor operates essentially as an isothermal backmix reactor (CSTR). What can you deduce about the apparent order of the reaction over the temperature range studied?
The plot gives the fractional decomposition of fed versus the ratio of reactor volume V (in cm3) to the NO2 feed rate, (g mol/h), at different feed concentrations of (in parts per million by weight). Determine as many rate law parameters as you can.
References
Citation
@online{utikar2024,
author = {Utikar, Ranjeet},
title = {Workshop 05: {Collection} and Analysis of Rate Data},
date = {2024-03-24},
url = {https://cre.smilelab.dev/content/workshops/05-collection-and-analysis-of-rate-data/},
langid = {en}
}