Solutions to workshop 08: Reaction mechanisms and catalysis
Lecture notes for chemical reaction engineering
Try following problems from Fogler 5e (Fogler (2016))
We will go through some of these problems in the workshop.
P 9-4, P 9-5, P 9-6, P 10-3, P 10-14, P 10-19
Solutions to these problems are uploaded at Workshop 8 solutions
P 9-4
The pyrolysis of acetaldehyde is believed to take place according to the following sequence:
Derive the rate expression for the rate of disappearance of acetaldehyde, .
Under what conditions does it reduce to the equation at the beginning of Section 9.1 on page 334?
Sketch a reaction pathway diagram for this reaction.
P 9-5
For each of the reactions in parts (a), (b), and (c), suggest a mechanism and apply the PSSH to learn if the mechanism is consistent with the rate law.
The gas-phase homogeneous oxidation of nitrogen monoxide () to dioxide ()
is known to have a form of third-order kinetics, which suggests that the reaction is elementary as written, at least for low partial pressures of the nitrogen oxides. However, the rate constant k actually decreases with increasing absolute temperature, indicating an apparently negative activation energy. Because the activation energy of any elementary reaction must be positive, some explanation is in order.
Provide an explanation, starting from the fact that an active intermediate species, NO 3 , is a participant in some other known reactions that involve oxides of nitrogen. Draw the reaction pathway.
The rate law for formation of phosgene, , from chlorine, , and carbon monoxide, , has the rate law
Suggest a mechanism for this reaction that is consistent with this rate law and draw the reaction pathway. Hint: Cl formed from the dissociation of is one of the two active intermediates.
Suggest an active intermediate(s) and mechanism for the reaction . Use the PSSH to show whether or not your mechanism is consistent with the rate law
P 9-6
(Tribology) Why you change your motor oil? One of the major reasons for engine-oil degradation is the oxidation of the motor oil. To retard the degradation process, most oils contain an antioxidant [see Ind. Eng. Chem. 26, 902 (1987)]. Without an inhibitor to oxidation present, the suggested mechanism at low temperatures is
where is an initiator and RH is the hydrocarbon in the oil.
When an antioxidant is added to retard degradation at low temperatures, the following additional termination steps occur:
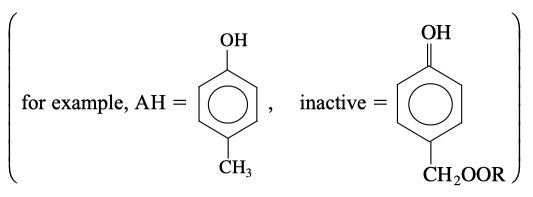
Derive a rate law for the degradation of the motor oil in the absence of an antioxidant at low temperatures.
Derive a rate law for the rate of degradation of the motor oil in the presence of an antioxidant for low temperatures.
How would your answer to part (a) change if the radicals were produced at a constant rate in the engine and then found their way into the oil?
Sketch a reaction pathway diagram for both high and low temperatures, with and without antioxidant.
P 10-3
t-Butyl alcohol (TBA) is an important octane enhancer that is used to replace lead additives in gasoline [Ind. Eng. Chem. Res., 27, 2224 (1988)]. TBA was produced by the liquid-phase hydration (W) of isobutene (I) over an Amberlyst-15 catalyst. The system is normally a multiphase mixture of hydrocarbon, water, and solid catalysts. However, the use of cosolvents or excess TBA can achieve reasonable miscibility.
The reaction mechanism is believed to be
Derive a rate law assuming:
The surface reaction is rate-limiting.
The adsorption of isobutene is limiting.
The reaction follows Eley–Rideal kinetics
and the surface reaction is limiting.
Isobutene (I) and water (W) are adsorbed on different sites.
TBA is not on the surface, and the surface reaction is rate-limiting.
What generalization can you make by comparing the rate laws derived in parts (a) through (d)?
P 10-14
Vanadium oxides are of interest for various sensor applications, owing to the sharp metal–insulator transitions they undergo as a function of temperature, pressure, or stress. Vanadium triisopropoxide (VTIPO) was used to grow vanadium oxide films by chemical vapor deposition [J. Electrochem. Soc., 136, 897 (1989)]. The deposition rate as a function of VTIPO pressure for two different temperatures follows.
T = 120 C:
| Growth Rate (m/h) | 0.004 | 0.015 | 0.025 | 0.04 | 0.068 | 0.08 | 0.095 | 0.1 | |
| VTIPO Pressure (torr) | 0.1 | 0.2 | 0.3 | 0.5 | 0.8 | 1.0 | 1.5 | 2.0 |
T = 200 C:
| Growth Rate (m/h) | 0.028 | 0.45 | 1.8 | 2.8 | 7.2 |
| VTIPO Pressure (torr) | 0.05 | 0.2 | 0.4 | 0.5 | 0.8 |
In light of the material presented in this chapter, analyze the data and describe your results. Specify where additional data should be taken.
P 10-19
With the increasing demand for xylene in the petrochemical industry, the production of xylene from toluene disproportionation has gained attention in recent years [Ind. Eng. Chem. Res., 26, 1854 (1987)]. This reaction,
was studied over a hydrogen mordenite catalyst that decays with time. As a first approximation, assume that the catalyst follows second-order decay
and the rate law for low conversions is
with and at .
Compare the conversion-time curves in a batch reactor containing 5 kg-cat at different initial partial pressures (1 atm, 10 atm, etc.). The reaction volume containing pure toluene initially is 1 and the temperature is 735 K.
What conversion can be achieved in a moving-bed reactor containing 50 kg of catalyst with a catalyst feed rate of 2 kg/h? Toluene is fed at a pressure of 2 atm and a rate of 10 mol/min.
Explore the effect of catalyst feed rate on conversion.
Suppose that and . What would the temperature–time trajectory look like for a CSTR? What if and ?
The decay law more closely follows the equation
with . Redo parts (b) and (c) for these conditions.
References
Citation
@online{utikar2024,
author = {Utikar, Ranjeet},
title = {Solutions to Workshop 08: {Reaction} Mechanisms and
Catalysis},
date = {2024-04-01},
url = {https://cre.smilelab.dev/content/workshops/08-reaction-mechanisms-and-catalysis/},
langid = {en}
}