<>:2: SyntaxWarning: invalid escape sequence '\ '
<>:2: SyntaxWarning: invalid escape sequence '\ '
C:\Users\Ranjeet\AppData\Local\Temp\ipykernel_47980\2700173291.py:2: SyntaxWarning: invalid escape sequence '\ '
df.columns = ['$X$', '$-r_A (mol/ kg-cat \ s)$']Portfolio 02: Conversion and reactor sizing
CHEN3010/ CHEN5040: chemical reaction engineering
Answers to the portfolio questions are uploaded at Portfolio 2 answers
Introduction
Ethylene oxide (EO) (), also known as oxirane, is a colorless, flammable gas with a sweet, ether-like smell. EO is an important industrial chemical used primarily to make ethylene glycol (a key ingredient in antifreeze and polyester fiber) and other chemicals, such as surfactants, detergents, and solvents.
In 2022, global production of EO was nearly 28 millions of tons and s expected to grow at a steady CAGR of 4.07% during the forecast period until 2032. With a selling price of $1913 per tonne, the commercial value of EO is around $53 billion.
The primary route for EO production involves the direct vapor phase oxidation of ethylene in the presence of a silver catalyst:
This reaction is highly exothermic and requires careful control to manage the risk of runaway reactions. The process typically involves a recovery stage to separate EO from water and other byproducts.
We want to design a reactor achieve a desired conversion of ethylene. Ethylene and oxygen are fed in stoichiometric proportions to a packed-bed reactor operated isothermally at 260 .
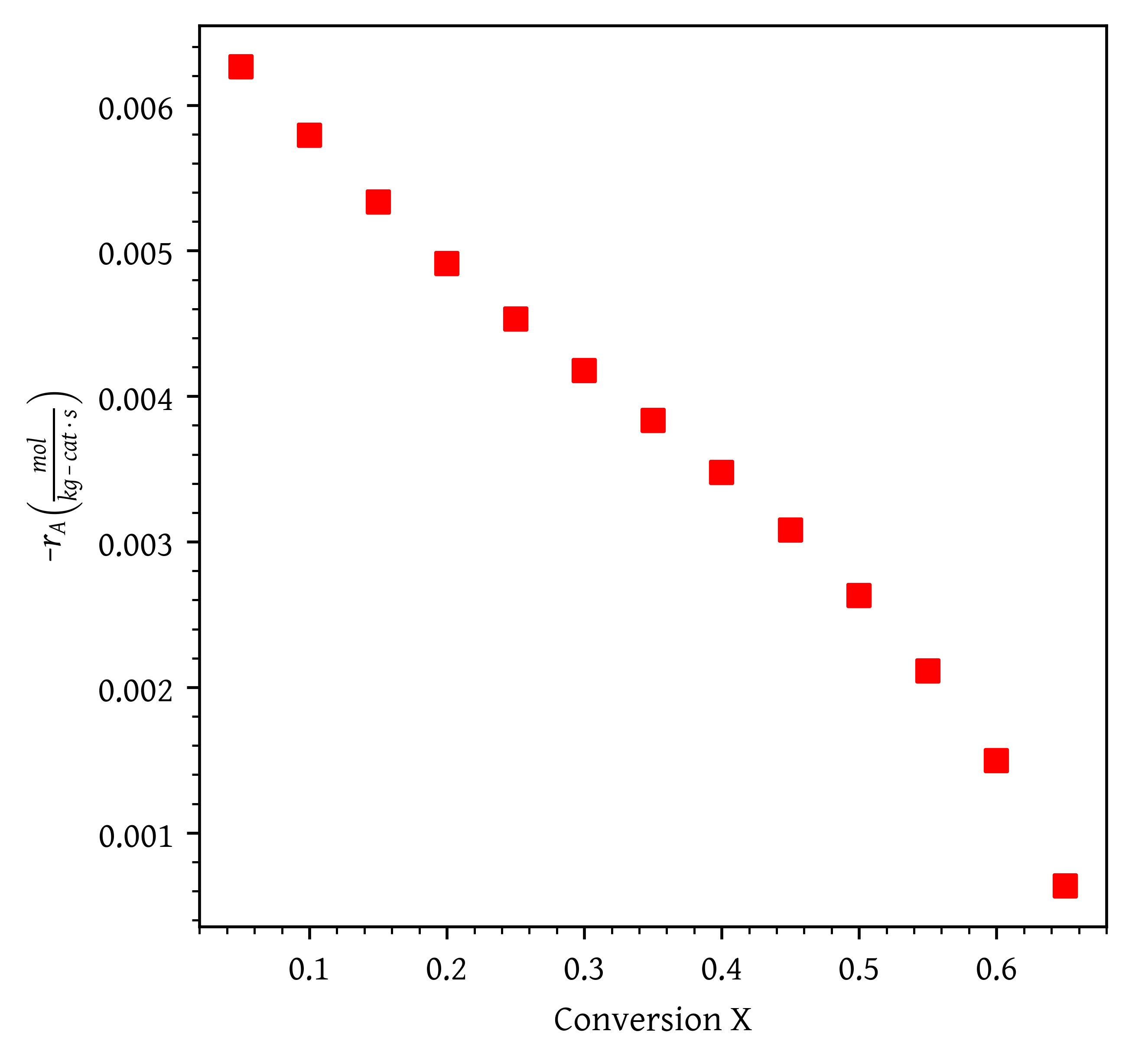
In order to aid reactor design, laboratory experiments were performed to determine reaction rate at various ethylene conversions. The reaction rate vs. conversion data (Table 1) is also provided in csv file ethylene_oxide_rate_vs_conversion.csv. The data is plotted in Figure 1.
Skeleton code useful for solving the questions in this portolio is provided in a Google colaboratory notebook
Question 1 (5 marks)
We want to achieve 60% conversion of ethylene. What kind of reactor would you suggest? Why?
<>:6: SyntaxWarning: invalid escape sequence '\c'
<>:6: SyntaxWarning: invalid escape sequence '\c'
C:\Users\Ranjeet\AppData\Local\Temp\ipykernel_47980\1231203049.py:6: SyntaxWarning: invalid escape sequence '\c'
ax.set_ylabel('$-r_A \\left(\\frac{mol}{kg-cat \cdot s} \\right)$')Question 2 (5 marks)
Write correct mole balance equation(s) for flow reactors in terms of conversion of ethylene.
Question 3 (10 marks)
Ethylene and oxygen are fed in stoichiometric proportions to a packed-bed reactor operated isothermally at 260 . Ethylene is fed at a rate of 136.21 mol/s at a pressure of 10 atm (1013 kPa). It is proposed to use 10 banks of 25.4 mm diameter schedule 40 tubes packed with catalyst with 100 tubes per bank. Consequently, the molar flow rate to each tube is to be 0.1362 mol/s.
The properties of the reacting fluid are to be considered identical to those of air at this temperature and pressure. The density of the 6.35 mm catalyst particles is 1925 , the bed void fraction is 0.45, and the gas density is 16 .
Determine the weight of catalyst required to achieve 60% conversion.
Question 4 (10 marks)
To improve temperature control, the suggestion is to conduct the reaction using two equally sized Continuous Stirred Tank Reactors (CSTRs) rather than a Packed Bed Reactor (PBR).
To achieve this, we equally divide the catalyst weight obtained in question 3 in to two CSTRs and join the CSTRs in series.
Calcualte the conversion attained at the end of second CSTR.
Citation
@online{untitled,
author = {},
title = {Portfolio 02: {Conversion} and Reactor Sizing},
url = {https://cre.smilelab.dev/content/portfolio/02-conversion-and-reactor-sizing/},
langid = {en}
}