<>:2: SyntaxWarning: invalid escape sequence '\ '
<>:2: SyntaxWarning: invalid escape sequence '\ '
C:\Users\Ranjeet\AppData\Local\Temp\ipykernel_37160\2700173291.py:2: SyntaxWarning: invalid escape sequence '\ '
df.columns = ['$X$', '$-r_A (mol/ kg-cat \ s)$']Portfolio 02: Conversion and reactor sizing
CHEN3010/ CHEN5040: chemical reaction engineering
General Instructions for in class Portfolios
- The portolio is an open-book task.
- You can use textbooks, the resources provided during class/ workshop etc. to answer the questions.
- The portfolio task is made available in both pdf format and as a print.
- You are free to choose a solution technique. It is not required that you use the provided python code to answer the questions. You can use any tool (pen and paper, excel, … ) and any technique (graphical, numerical, analytical) that you are comfortable with.
- Irrespective of your solution method, you are expected to write your answers on to the printed question paper provided. This is what gets marked.
- The portfolio will take place during designated timeslot communicated earlier by the unit coordinator. Please refer to the portfolio schedule on blackboard for the portolio dates and topics.
- The tasks will be a mix of theory questions, short calculation type and long numerical examples.
- You have 30 minutes to complete the tasks in the portfolio.
- The portolios will be marked immediately after completion by your peers using a provided marking rubric.
- The portfolios will be collected by the instructors to verify peer marking and record the marks. You will receive your portolio back within a week.
- When you are required to upload the portolio answers on to blackboard:
- Save your report as a pdf file.
- Rename the file as STUDENTID_Portfolio_x.pdf (Where STUDENTID is your student ID, and x is the portfolio number) and
- Upload it using assessment submission link on blackboard.
Academic Integrity
Academic integrity at its core is about honesty and responsibility and is fundamental to Curtin’s expectations of you. This means that all of your work at Curtin should be your own and it should be underpinned by integrity, which means to act ethically, honestly and with fairness.
As a Curtin student you are part of an academic community and you are asked to uphold the University’s Code of Conduct, principles of academic integrity, and Curtin’s five core values of integrity, respect, courage, excellence and impact during your studies.
You are also expected to uphold the Student Charter and recognise that cheating, plagiarism collusion, and falsification of data and other forms of academic dishonesty are not acceptable.
For more information, visit https://students.curtin.edu.au/essentials/rights/academic-integrity/
Introduction
Ethylene oxide (EO) (), also known as oxirane, is a colorless, flammable gas with a sweet, ether-like smell. EO is an important industrial chemical used primarily to make ethylene glycol (a key ingredient in antifreeze and polyester fiber) and other chemicals, such as surfactants, detergents, and solvents.
In 2022, global production of EO was nearly 28 millions of tons and s expected to grow at a steady CAGR of 4.07% during the forecast period until 2032. With a selling price of $1913 per tonne, the commercial value of EO is around $53 billion.
The primary route for EO production involves the direct vapor phase oxidation of ethylene in the presence of a silver catalyst:
This reaction is highly exothermic and requires careful control to manage the risk of runaway reactions. The process typically involves a recovery stage to separate EO from water and other byproducts.
We want to design a reactor achieve a desired conversion of ethylene. Ethylene and oxygen are fed in stoichiometric proportions to a packed-bed reactor operated isothermally at 260 .
In order to aid reactor design, laboratory experiments were performed to determine reaction rate at various ethylene conversions.
Skeleton code useful for solving the questions in this portolio is provided in a Google colaboratory notebook
Question 1 (5 marks)
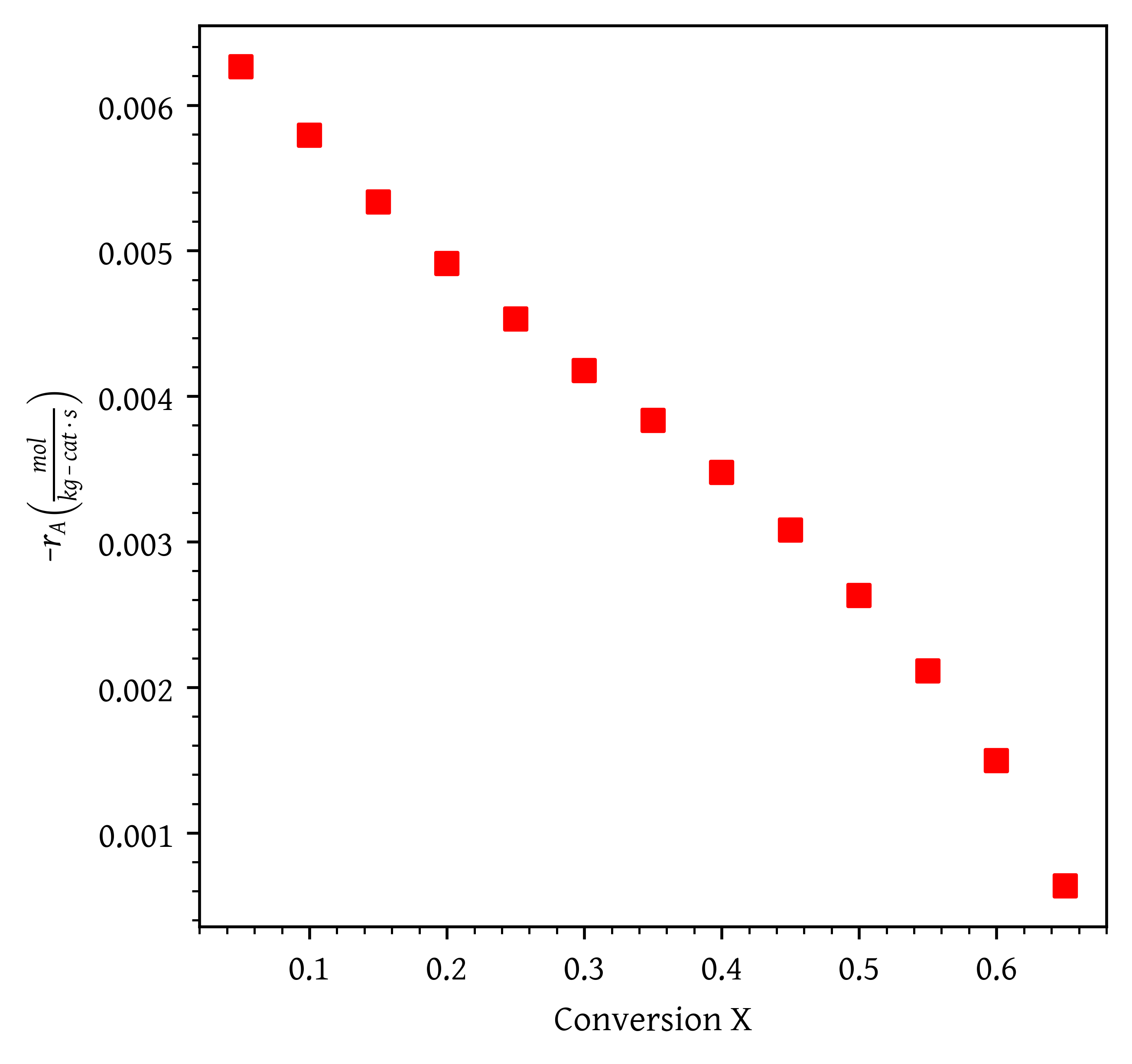
The reaction rate vs. conversion data is provided in csv file ethylene_oxide_rate_vs_conversion.csv. The data is plotted in Figure 1. We want to achieve 60% conversion of ethylene. What kind of reactor would you suggest? Why?
<>:6: SyntaxWarning: invalid escape sequence '\c'
<>:6: SyntaxWarning: invalid escape sequence '\c'
C:\Users\Ranjeet\AppData\Local\Temp\ipykernel_37160\1231203049.py:6: SyntaxWarning: invalid escape sequence '\c'
ax.set_ylabel('$-r_A \\left(\\frac{mol}{kg-cat \cdot s} \\right)$')Answer
Since this is a gas phase catalytic reaction a packed bed reactor would be ideal. (2 marks)
The graph is monotonously decreasing with conversion. Hence increases monotonically with conversion.
The amount of catalyst required is the area under the curve for a PBR and the area of rectangle with a width of and height of .
For a monotonously increasing curve, catalyst weight using CSTR will always be higher than a PBR. Therefore PBR is preferred. (3 marks)
Question 2 (5 marks)
Write correct mole balance equation(s) for flow reactors in terms of conversion of ethylene.
Answer
We write mole balance equations for the two flow reactors: CSTR, and PBR
CSTR (2 marks)
PBR
(2 marks)
and
(1 mark)
or
Question 3 (10 marks)
Ethylene and oxygen are fed in stoichiometric proportions to a packed-bed reactor operated isothermally at 260 . Ethylene is fed at a rate of 136.21 mol/s at a pressure of 10 atm (1013 kPa). It is proposed to use 10 banks of 25.4 mm diameter schedule 40 tubes packed with catalyst with 100 tubes per bank. Consequently, the molar flow rate to each tube is to be 0.1362 mol/s.
The properties of the reacting fluid are to be considered identical to those of air at this temperature and pressure. The density of the 6.35 mm catalyst particles is 1925 , the bed void fraction is 0.45, and the gas density is 16 .
Determine the weight of catalyst required to achieve 60% conversion.
Answer
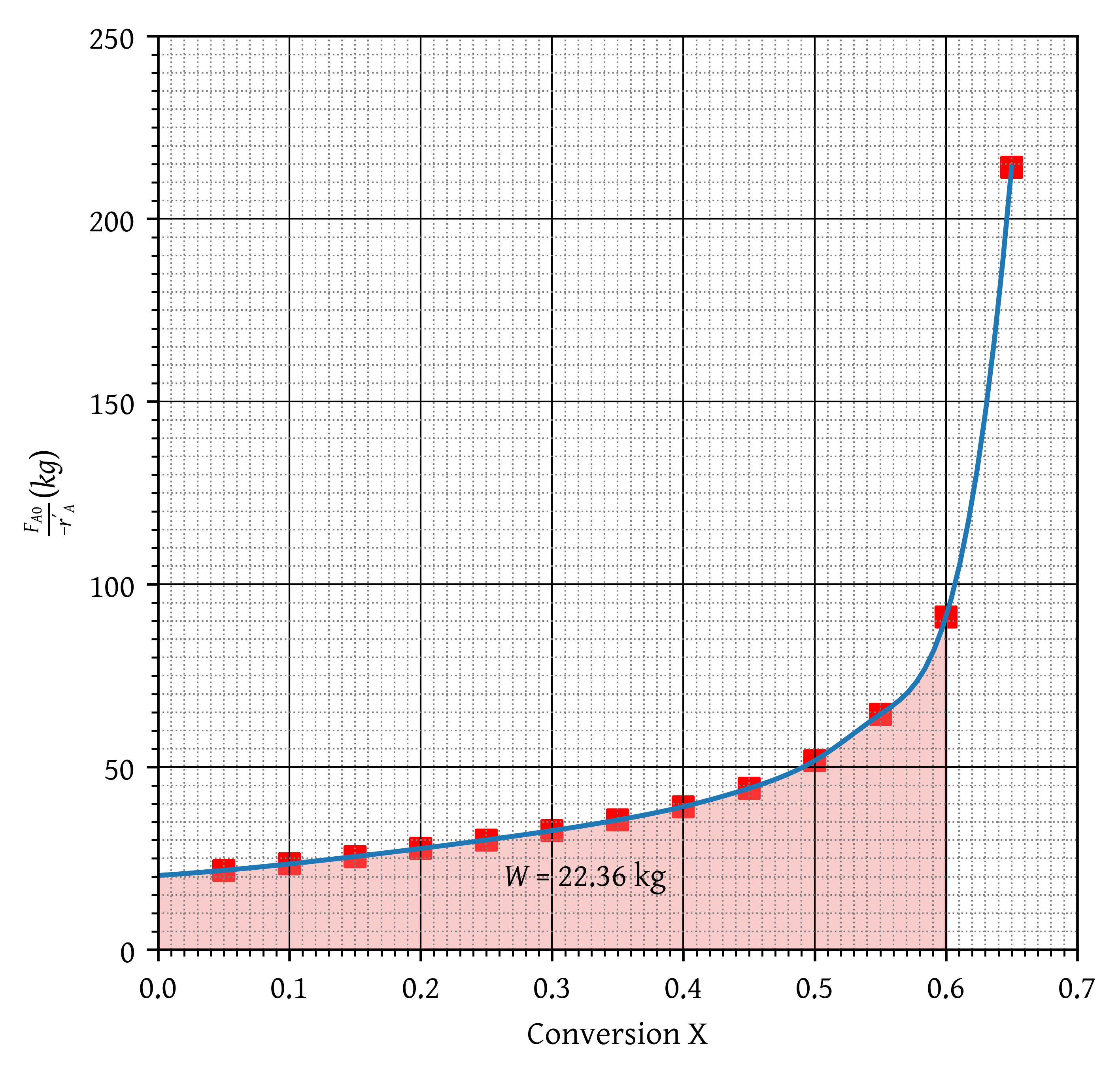
Steps
- Correctly calculates (). (2 marks)
- A plot of is drawn correctly. (2 marks)
- X and Y axis labels and units are correct. (2 marks)
- PBR catalyst weight is denoted (shaded area under curve). (2 marks)
- Correct catalyst weight is reported. (2 marks for W around 22.36 kg)
- half marks for essentially correct procedure but wrong answers.
Question 4 (10 marks)
To improve temperature control, the suggestion is to conduct the reaction using two equally sized Continuous Stirred Tank Reactors (CSTRs) rather than a Packed Bed Reactor (PBR).
To achieve this, we equally divide the catalyst weight obtained in question 3 in to two CSTRs and join the CSTRs in series.
Calcualte the conversion attained at the end of second CSTR.
Answer
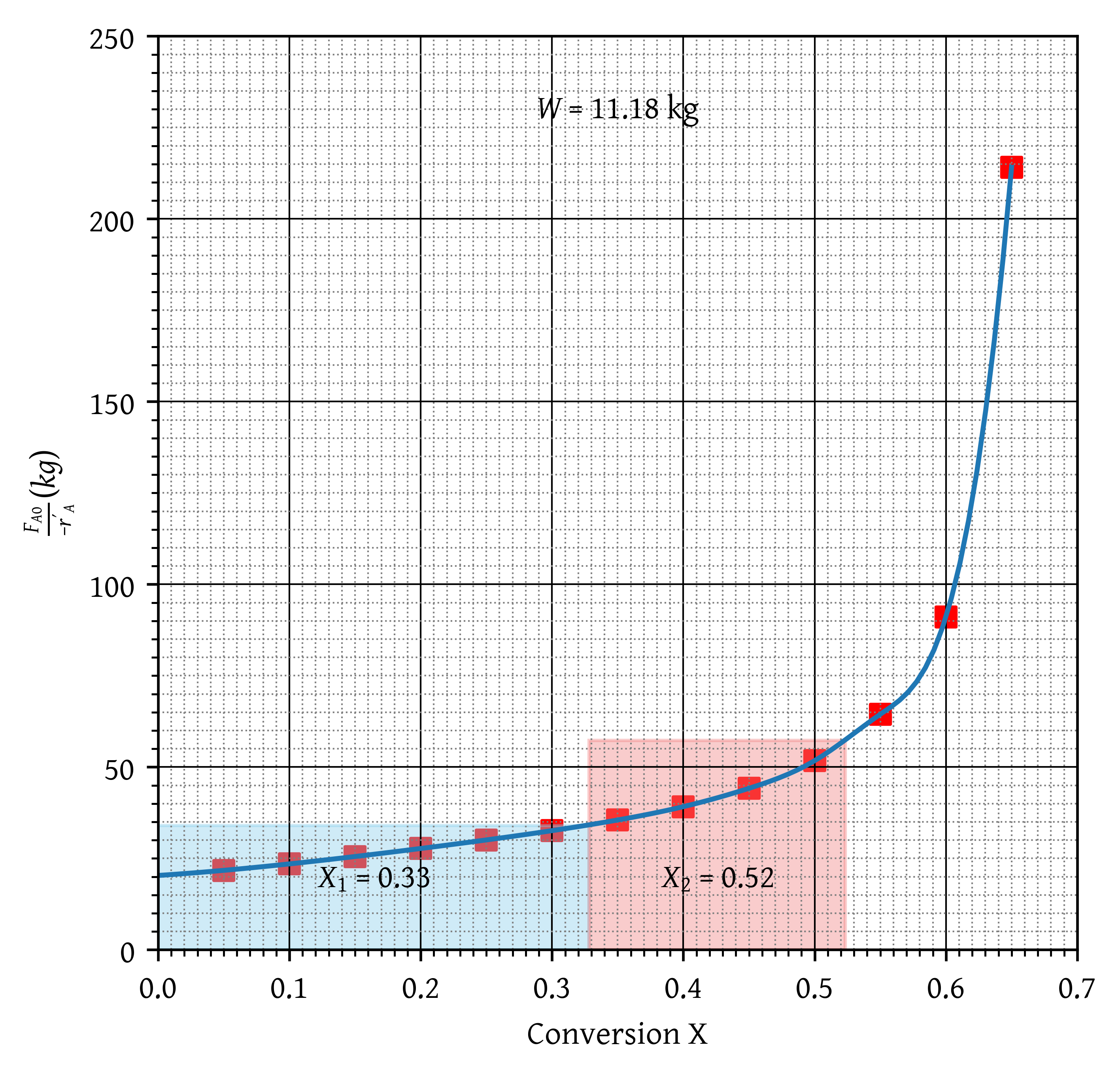
Steps
- A plot of is drawn and labelled correctly. (2 marks)
- CSTR catalyst weight per reactor (11.18 kg) is determined. (2 marks)
- CSTR catalyst weight is denoted (shaded area of rectangle for reactor 1 and 2). (2 marks)
- Correct conversion is reported for reactor 1. (2 marks for X around 0.33)
- Correct conversion is reported for reactor 2. (2 marks for X around 0.52)
- half marks for essentially correct procedure but wrong answers.
Citation
@online{untitled,
author = {},
title = {Portfolio 02: {Conversion} and Reactor Sizing},
url = {https://cre.smilelab.dev/content/portfolio/02-conversion-and-reactor-sizing/portfolio-02-answers.html},
langid = {en}
}