import math
# Data given
k = 0.23 # 1/min
Ca0_by_Ca = 1/ 0.1
upsilon_0 = 10 # dm^3/min
V = (upsilon_0/k)* math.log(Ca0_by_Ca)In class activity: Mole balances
Lecture notes for chemical reaction engineering
Problems
Chloral is being consumed at a rate of 10 moles per second per m3 when reacting with chlorobenzene to form DDT and water in the reaction described above. In symbol form, the reaction is written as
Write the rates of disappearance and formation (i.e., generation) for each species in this reaction.
Solution- Rate of disappearance of A: 10
- Rate of disappearance of B: 2*10 = 20
- Rate of formation of C: 10
- Rate of formation of D: 10
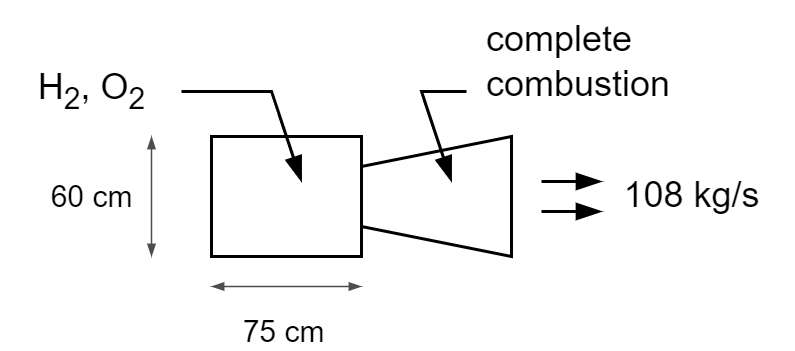
A rocket engine, burns a stoichiometric mixture of fuel (liquid hydrogen) in oxidant (liquid oxygen). The combustion chamber is cylindrical, 75 cm long and 60 cm in diameter, and the combustion process produces 108 kg/s of exhaust gases. If combustion is complete, find the rate of reaction of hydrogen and of oxygen.
Solution= 0.2121
Reactions occuring:
Molecular weights: ; ;
- produced: 108/18 = 6 kmol/s
- used: 6 kmol/s
- used: 3 kmol/s
A human being (75 kg) consumes about 6000 kJ of food per day. Assume that I the food is all glucose and that the overall reaction is
Find man’s metabolic rate (the rate of living, loving, and laughing) in terms of moles of oxygen used per m3 of person per second. Assume average density of a human being to be .
SolutionWe want to find
Each mole of glucose consumed uses 6 moles of oxygen and releases 2816 kJ of energy
Consider the liquid phase cis – trans isomerization of 2–butene which we will write symbolically as
The reaction is first order in A () and is carried out in a tubular reactor in which the volumetric flow rate,, is constant, i.e.,.
- Sketch the concentration profile.
- Derive an equation relating the reactor volume to the entering and exiting concentrations of A, the rate constant k, and the volumetric flow rate.
- Determine the reactor volume, , necessary to reduce the exiting concentration to 10% of the entering concentration, i.e., , when the volumetric flow rate is (i.e., liters/min) and the specific reaction rate, k, is .
SolutionConcentration Profile
PFR concentration profile schematic Rate equation:
PFR mole balance:
= 100.11
Citation
@online{utikar2024,
author = {Utikar, Ranjeet},
title = {In Class Activity: {Mole} Balances},
date = {2024-02-22},
url = {https://cre.smilelab.dev/content/notes/01-mole-balances/in-class-activities.html},
langid = {en}
}