import numpy as np
from scipy.integrate import solve_ivp
from scipy.optimize import fsolve
import matplotlib.pyplot as plt
from IPython.display import display, Markdown
def batch_reactor(t, y, *args):
ca, cd, cu = y
k1, k2, k1A, k2A = args
r1a = k1 * (ca - cd/k1A)
r2a = k2 * (ca - cu/k2A)
dcadt = -r1a -r2a
dcddt = r1a
dcudt = r2a
return [dcadt, dcddt, dcudt]
k1 = 1.0 # 1/min
k2 = 100 # 1/min
k1A = 10.0
k2A = 1.5
ca0 = 1.0
# initial conditions
y0 = [ca0, 0, 0]
args = (k1, k2, k1A, k2A)
t_final = 12
sol = solve_ivp(batch_reactor, [0, t_final], y0, args=args, dense_output=True)
t = np.linspace(0,t_final, 1000)
ca, cd, cu = sol.sol(t)Solutions to workshop 06: Multiple reactions
Lecture notes for chemical reaction engineering
Try following problems from Fogler 5e (Fogler 2016). P 8-3, P 8-4, P 8-7, P 8-9
We will go through some of these problems in the workshop.
P 8-3
The following reactions
take place in a batch reactor.
Additional information:
= 1.0 min–1; = 10
= 100 min–1; = 1.5
= 1 mol/dm3
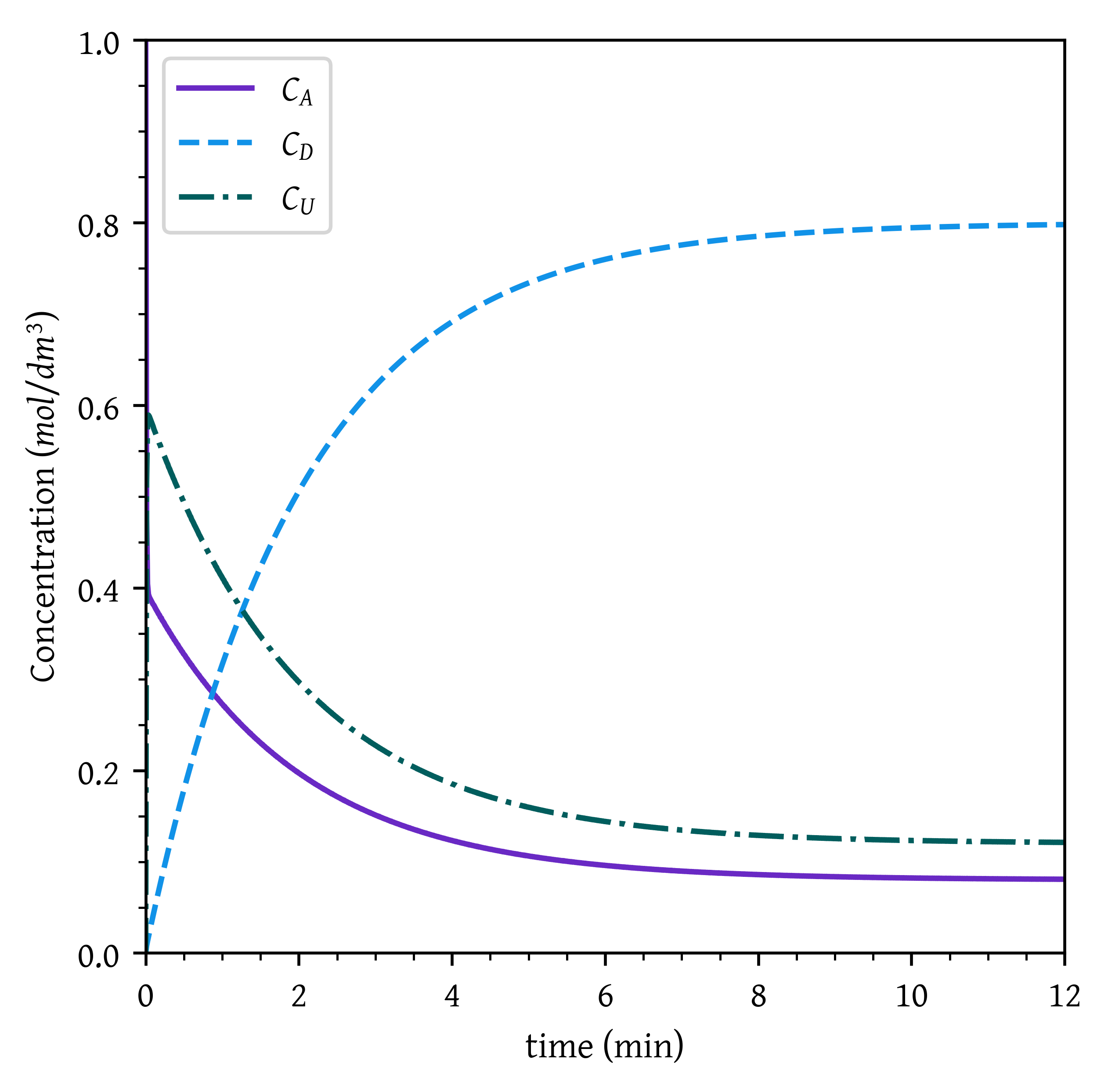
Plot and analyze conversion and the concentrations of A, D, and U as a function of time. When would you stop the reaction to maximize the concentration of D? Describe what you find.
When does the maximum concentration of U occur? (Ans.: t = 0.04 min)
What are the equilibrium concentrations of A, D, and U?
What would be the exit concentrations from a CSTR with a space time of 1.0 min? Of 10.0 min? Of 100 min?
plt.plot(t, ca, label='$C_A$')
plt.plot(t, cd, label='$C_D$')
plt.plot(t, cu, label='$C_U$')
plt.xlabel('time (min)')
plt.ylabel('Concentration ($mol/dm^3$)')
# Setting x and y axis limits
plt.xlim(0, t_final)
plt.ylim(0, ca0)
plt.legend()
plt.show()cumax_idx = np.argmax(cu)
tumax = t[cumax_idx]
cumax = cu[cumax_idx]Maximum concentration of U, = = 0.59 occurs at = 0.04 min
As keeps on increasing with time until the equilibrium is reached, To maximize , stop the reaction after equilibrium is reached.
cae = ca[-1]
cde = cd[-1]
cue = cu[-1]Equilibrium concentrations
= 0.08
= 0.80
= 0.12
def cstr(vars, *args):
ca, cd, cu = vars
t, k1, k2, k1A, k2A = args
r1a = k1 * (ca - cd/k1A)
r2a = k2 * (ca - cu/k2A)
eq1 = ca0 - ca - t * (r1a + r2a)
eq2 = -cd + t * r1a
eq3 = -cu + t * r2a
return [eq1, eq2, eq3]
initial_guess = [ca0, 0, 0]
times = [1, 10, 100]
md = ""
for tau in times:
args = (tau, k1, k2, k1A, k2A)
ca, cd, cu = fsolve(cstr, initial_guess, args=args)
md += (
f"At $\\tau$ = {tau:.2f} min.:\n"
f"$C_A$ = {ca:.2f} $mol/dm^3$,\n"
f"$C_D$ = {cd:.2f} $mol/dm^3$,\n"
f"$C_U$ = {cu:.2f} $mol/dm^3$ \n\n"
)
display(Markdown(md))At = 1.00 min.: = 0.30 , = 0.27 , = 0.44
At = 10.00 min.: = 0.13 , = 0.67 , = 0.20
At = 100.00 min.: = 0.09 , = 0.78 , = 0.13
P 8-4
Consider the following system of gas-phase reactions:
B is the desired product, and X and Y are foul pollutants that are expensive to get rid of. The specific reaction rates are at 27 C. The reaction system is to be operated at 27 C and 4 atm. Pure A enters the system at a volumetric flow rate of 10 dm3/min.
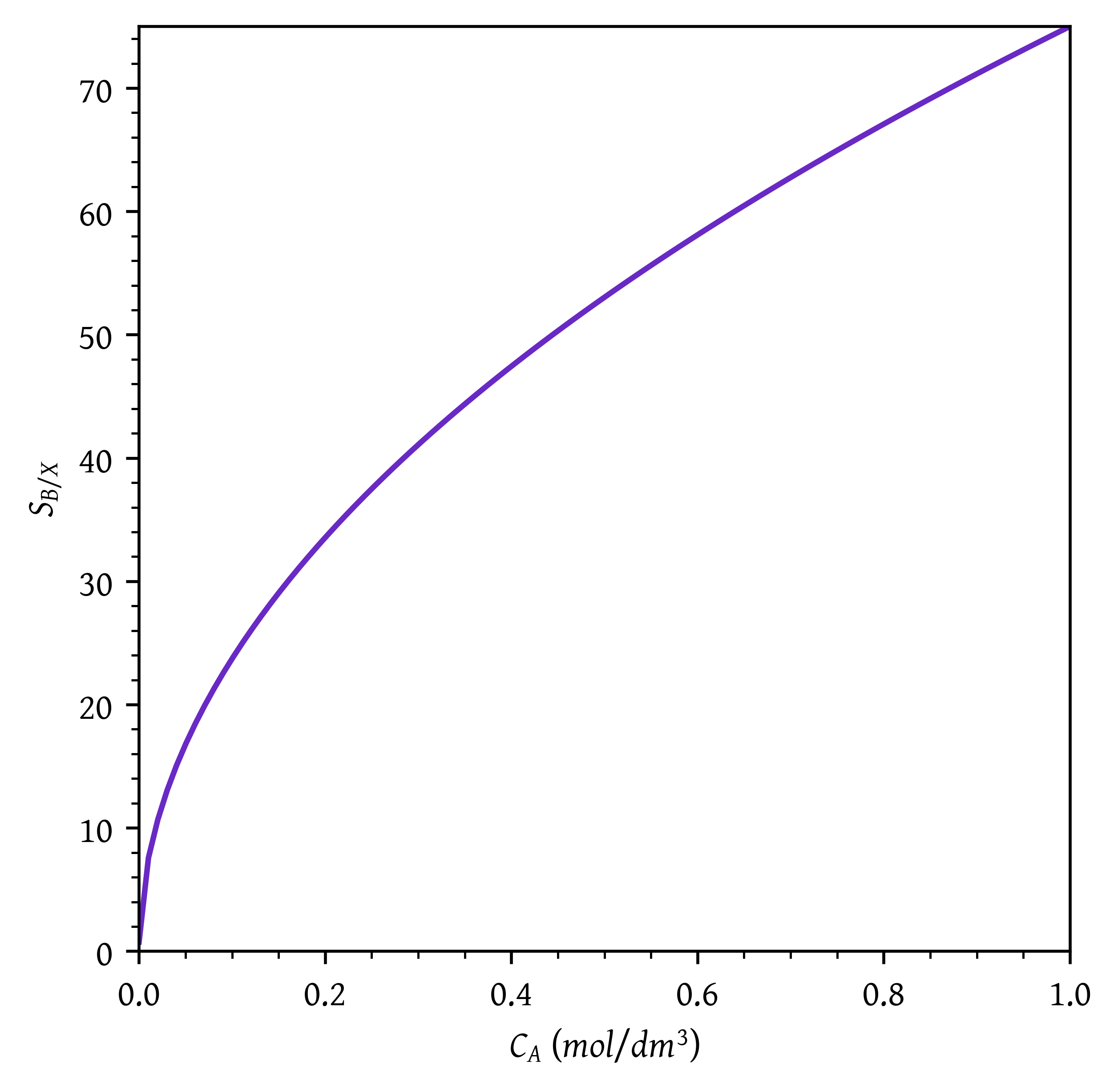
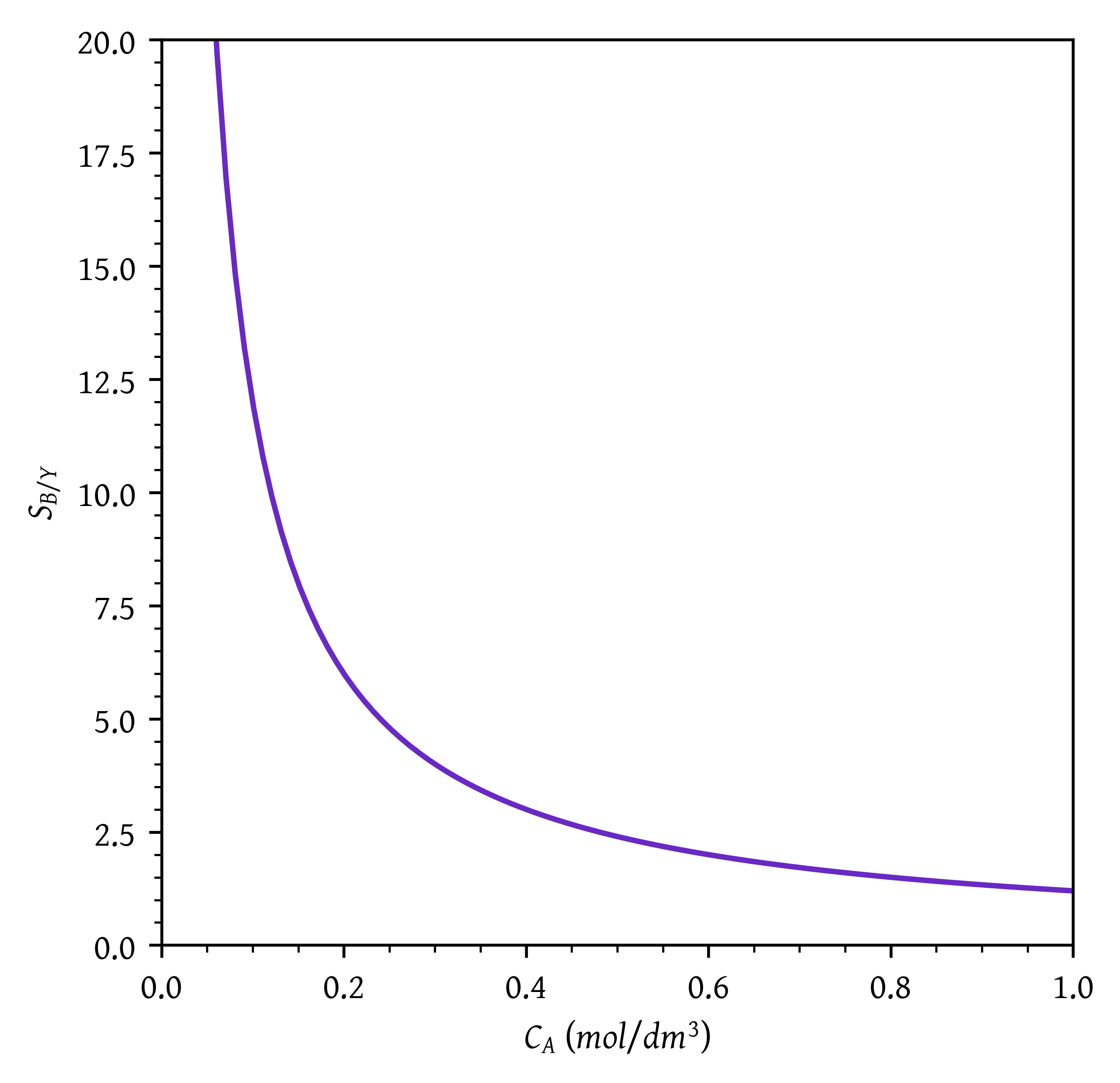
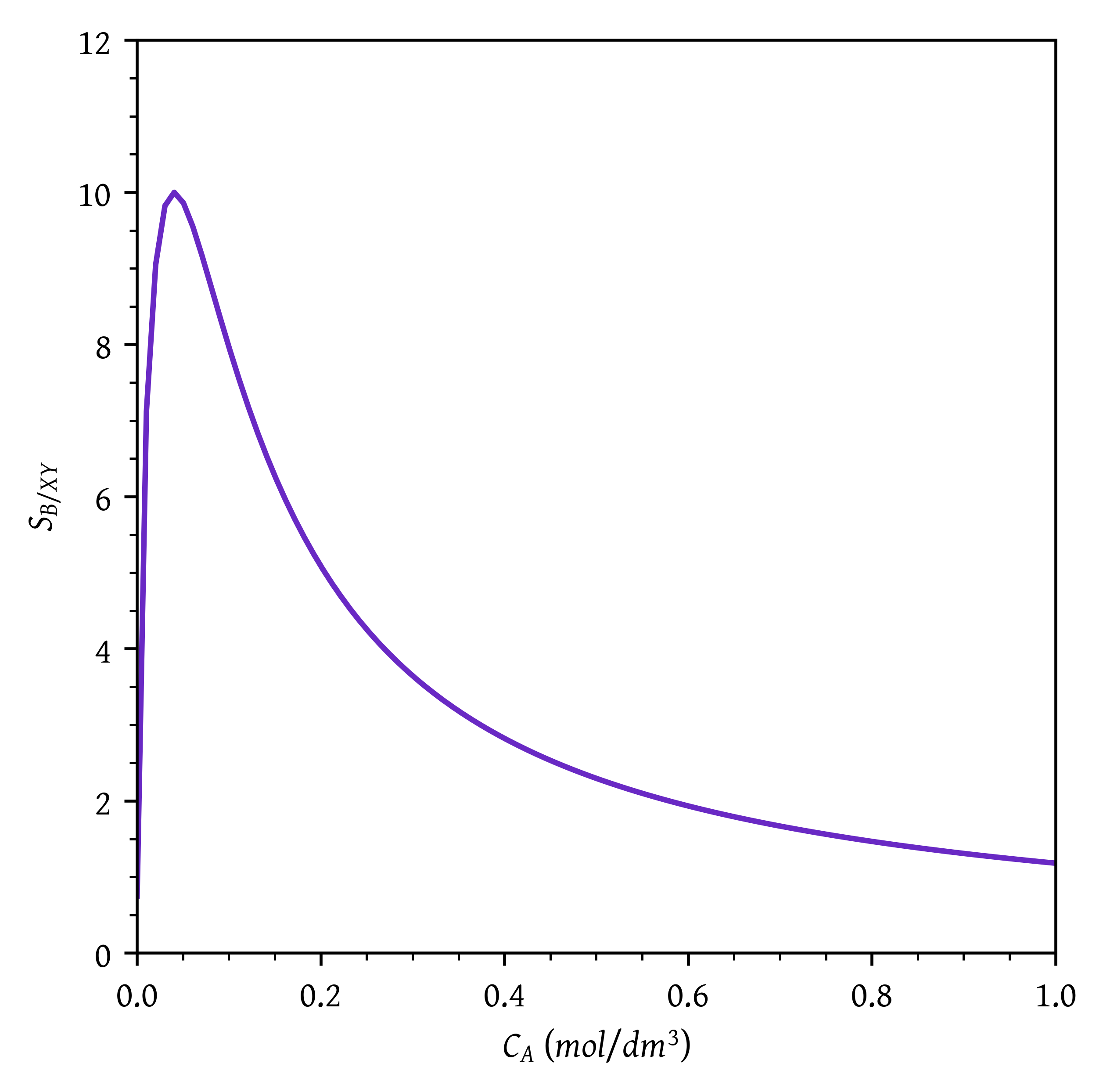
Sketch the instantaneous selectivities as a function of the concentration of CA.
Consider a series of reactors. What should be the volume of the first reactor?
What are the effluent concentrations of A, B, X, and Y from the first reactor?
What is the conversion of A in the first reactor?
If 99% conversion of A is desired, what reaction scheme and reactor sizes should you use to maximize ?
Suppose that E1 = 20,000 cal/mol, E2=10,000 cal/mol, and E3=30,000 cal/mol. What temperature would you recommend for a single CSTR with a space time of 10 min and an entering concentration of A of 0.1 mol/dm3 ?
import numpy as np
from scipy.integrate import solve_ivp
from scipy.optimize import fsolve
from scipy.optimize import minimize_scalar
from scipy.integrate import quad
import matplotlib.pyplot as plt
from IPython.display import display, Markdown
rx = lambda ca: 0.004 * ca**0.5
rb = lambda ca: 0.3 * ca
ry = lambda ca: 0.25 * ca**2
sbx = lambda ca: rb(ca)/rx(ca)
sby = lambda ca: rb(ca)/ry(ca)
sbxy = lambda ca: rb(ca)/(rx(ca) + ry(ca))
ca = np.linspace(1e-4,1,100)plt.plot(ca,sbx(ca))
plt.xlabel('$C_A$ ($mol/dm^3$)')
plt.ylabel('$S_{B/X}$')
# Setting x and y axis limits
plt.xlim(0, ca[-1])
plt.ylim(0, np.ceil(sbx(ca[-1])))
plt.show()plt.plot(ca,sby(ca))
plt.xlabel('$C_A$ ($mol/dm^3$)')
plt.ylabel('$S_{B/Y}$')
# Setting x and y axis limits
plt.xlim(0, ca[-1])
plt.ylim(0, 20)
plt.show()plt.plot(ca,sbxy(ca))
plt.xlabel('$C_A$ ($mol/dm^3$)')
plt.ylabel('$S_{B/XY}$')
# Setting x and y axis limits
plt.xlim(0, ca[-1])
plt.ylim(0, 12)
plt.show()# Maximize S_B/XY
bounds=(1e-4, 1)
# Maximize the selectivity by minimizing the negative of the selectivity function
result = minimize_scalar(lambda ca: -sbxy(ca), method='bounded', bounds=bounds)
camax = result.x
sbxy_max = sbxy(camax)Maximum concentration of A, = 0.04
Maximum selectivity = 10.00
The CSTR operates at exit concentration of 0.04
T = 27 + 273.15 # K
v0 = 10 # dm^3/min
P = 4 # atm
ya0 = 1 # pure A
R = 0.082
ca0 = ya0 * P /(R * T)
rate = rx(camax) + rb(camax) + ry(camax)
v_cstr = v0 * (ca0 - camax)/ rate- = 0.163
- = 92.81
tau_cstr = v_cstr/v0
cb_cstr = tau_cstr* rb(camax)
cx_cstr = tau_cstr* rx(camax)
cy_cstr = tau_cstr* ry(camax)Exit concentration of CSTR
- = 1.114e-01
- = 7.425e-03
- = 3.713e-03
x_cstr = (ca0 - camax)/ca0Conversion in first reactor = 0.75
fa0 = ca0*v0
fa0_by_ra = fa0/(rb(ca) + rx(ca) + ry(ca))
x = (ca0 - ca)/ca0
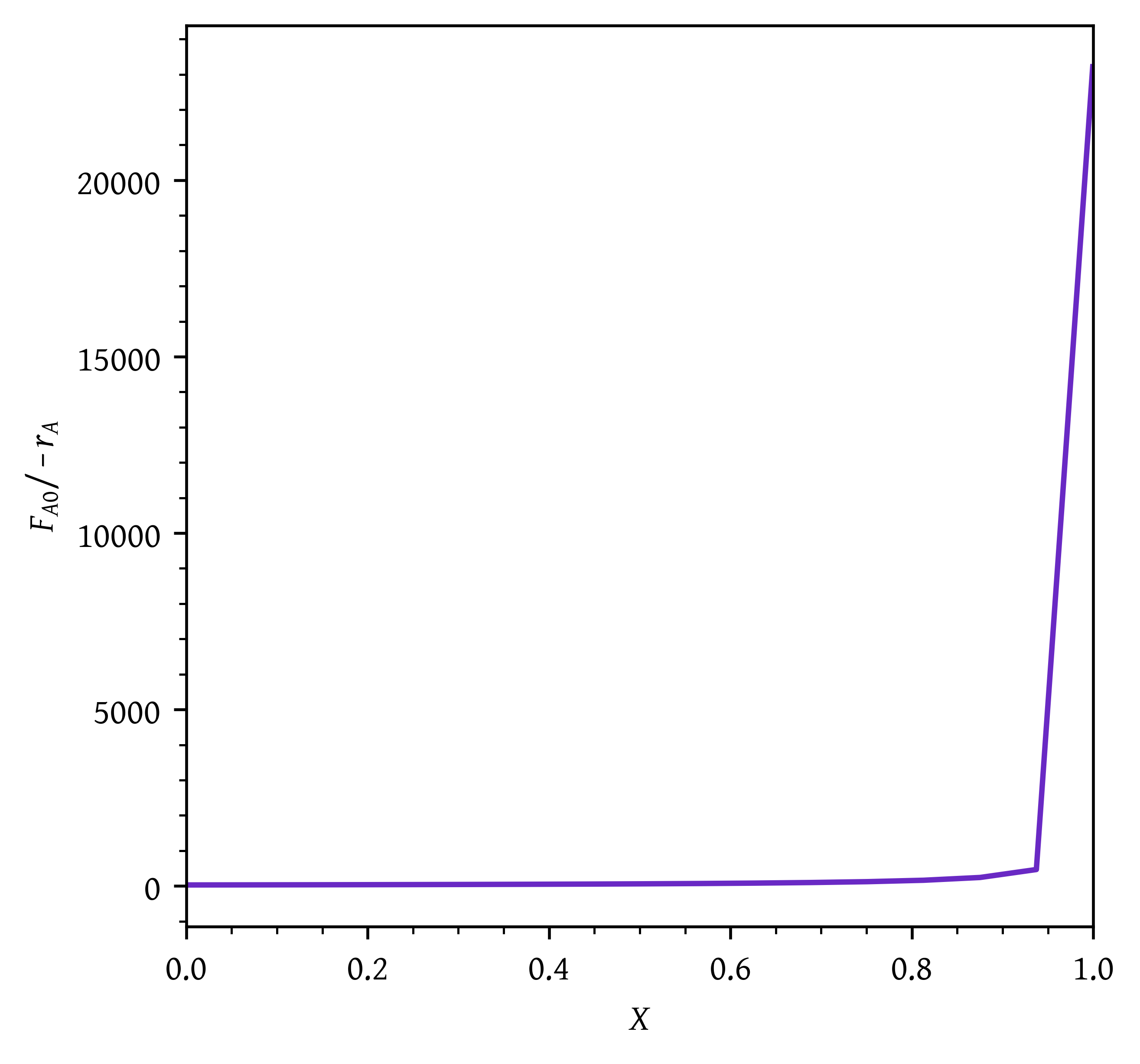
plt.plot(x,fa0_by_ra)
plt.xlabel('$X$')
plt.ylabel('$F_{A0}/-r_A$')
# Setting x and y axis limits
plt.xlim(0, 1)
# plt.ylim(0, 10000)
plt.show()def pfr(x, *args):
fa0 = args[0]
ca = ca0 * (1 - x)
return fa0/(rb(ca) + rx(ca) + ry(ca))
x_start = x_cstr
x_end = 0.99
v_pfr, _ = quad(pfr, x_start, x_end, args=(fa0))PFR volume required to achieve final conversion of 0.99 = 91.18
e1 = 20000
e2 = 10000
e3 = 30000
k1 = lambda T: 0.004 * np.exp((e1/1.98) * ((1/T) - (1/300)))
k2 = lambda T: 0.3 * np.exp((e2/1.98) * ((1/T) - (1/300)))
k3 = lambda T: 0.25 * np.exp((e3/1.98) * ((1/T) - (1/300)))
rxt = lambda ca,T: k1(T) * ca**0.5
rbt = lambda ca,T: k2(T) * ca
ryt = lambda ca,T: k3(T) * ca**2
sbxt = lambda ca,T: rbt(ca,T)/rxt(ca,T)
sbyt = lambda ca,T: rbt(ca,T)/ryt(ca,T)
sbxyt = lambda ca,T: rbt(ca,T)/(rxt(ca,T) + ryt(ca,T))
tau = 10
ca0 = 0.1
initial_guess = [ca0, 0, 0, 0]
def cstr_btx(vars, *args):
ca, cx, cb, cy = vars
tau, T = args
r1 = rxt(ca,T)
r2 = rbt(ca,T)
r3 = ryt(ca,T)
eq1 = ca0 - ca - tau * (r1 + r2 + r3)
eq2 = -cx + tau * r1
eq3 = -cb + tau * r2
eq4 = -cy + tau * r3
return [eq1, eq2, eq3, eq4]
# Function to solve the CSTR equations for a given temperature
def maximize_cb(T):
args = (tau, T)
ca, cx, cb, cy = fsolve(cstr_btx, initial_guess, args=args)
return -cb
result = minimize_scalar(maximize_cb, bounds=(275, 400), method='bounded')
optimal_temp = result.x
max_cb = -result.fun
temps = np.linspace(275,400,100)
cb_values = []
for temp in temps:
args = (tau, temp)
ca, cx, cb, cy = fsolve(cstr_btx, initial_guess, args=args)
cb_values.append(cb)
cb_values = np.array(cb_values)
temps = np.array(temps)
max_cb_index = np.argmax(cb_values)
max_cb_temp = temps[max_cb_index]
max_cb = cb_values[max_cb_index]
plt.plot(temps, cb_values)
plt.scatter([max_cb_temp], [max_cb], zorder=5)
# plt.axvline(x=max_cb_temp, color='gray', linestyle='--', lw=1)
# plt.axhline(y=max_cb, color='gray', linestyle='--', lw=1)
plt.plot([max_cb_temp, max_cb_temp], [0, max_cb], color='gray', linestyle='--', lw=1) # Vertical line to curve
plt.plot([min(temps), max_cb_temp], [max_cb, max_cb], color='gray', linestyle='--', lw=1) # Horizontal line to curve
plt.xlabel('Temperature (K)')
plt.ylabel('Concentration of B ($mol/dm^3$)')
# Setting x and y axis limits
plt.xlim(275, 400)
plt.ylim(0, 0.08)
plt.show()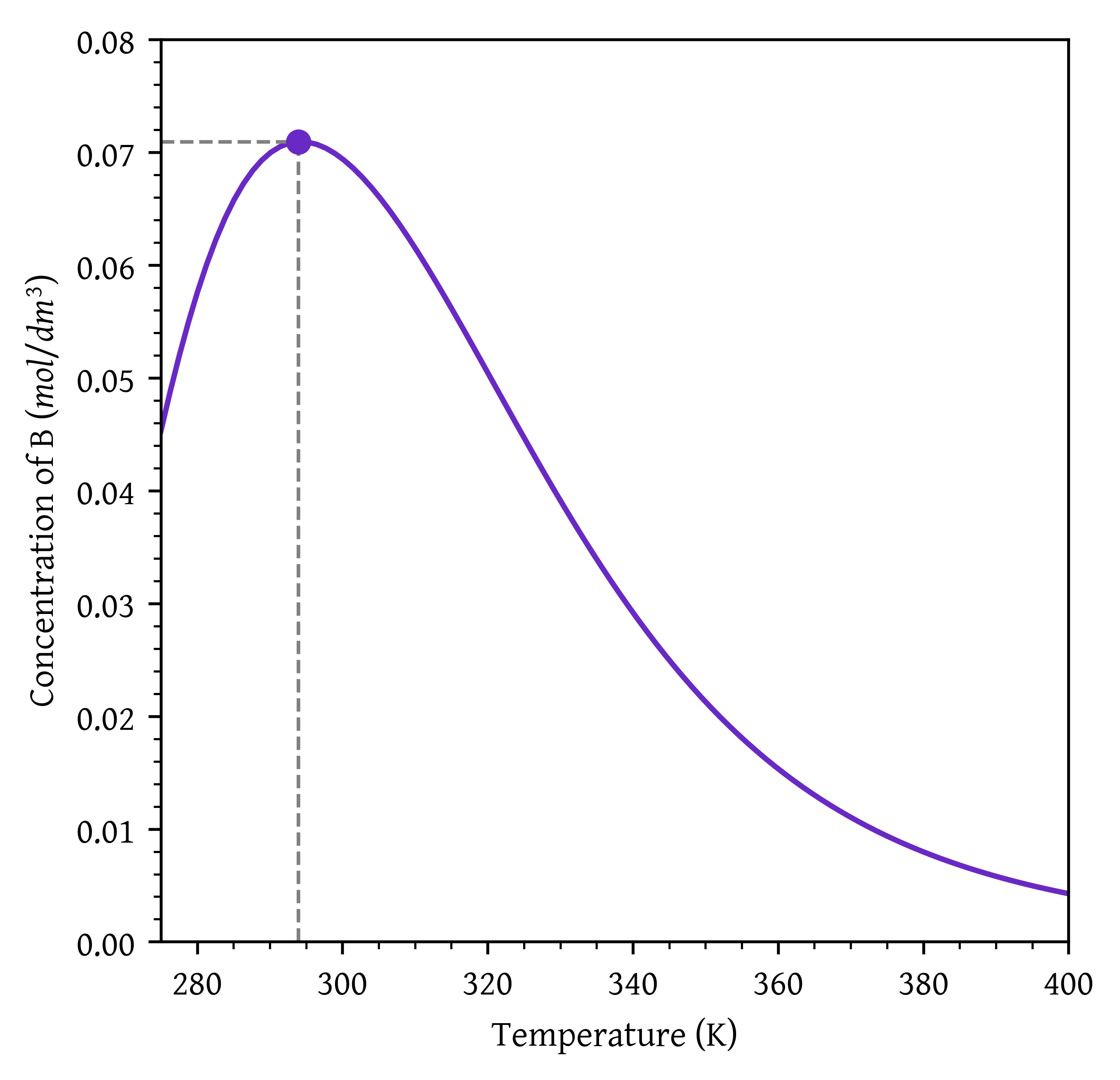
- Optimal temperature for maximizing = 294.26 K. Maximum = 0.07
P 8-9
The elementary liquid-phase series reaction
is carried out in a 500-dm3 batch reactor. The initial concentration of A is 1.6 mol/dm3. The desired product is B, and separation of the undesired product C is very difficult and costly. Because the reaction is carried out at a relatively high temperature, the reaction is easily quenched.
Plot and analyze the concentrations of A, B, and C as a function of time. Assume that each reaction is irreversible, with and .
Plot and analyze the concentrations of A, B, and C as a function of time when the first reaction is reversible, with .
Plot and analyze the concentrations of A, B, and C as a function of time for the case where both reactions are reversible, with .
Compare (a), (b), and (c) and describe what you find.
Vary . Explain the consequence of and and with and with , and .
import numpy as np
from scipy.integrate import solve_ivp
import matplotlib.pyplot as plt
def batch_reactor_a(t, y, *args):
ca, cb, cc = y
k1, k2 = args
r1a = k1 * ca
r2b = k2 * cb
dcadt = - r1a
dcbdt = r1a - r2b
dccdt = r2b
return [dcadt, dcbdt, dccdt]
k1 = 0.4 # 1/h
k2 = 0.01
ca0 = 1.6
# initial conditions
y0 = [ca0, 0, 0]
args = (k1, k2)
t_final = 50
sol = solve_ivp(batch_reactor_a, [0, t_final], y0, args=args, dense_output=True)
t = np.linspace(0,t_final, 1000)
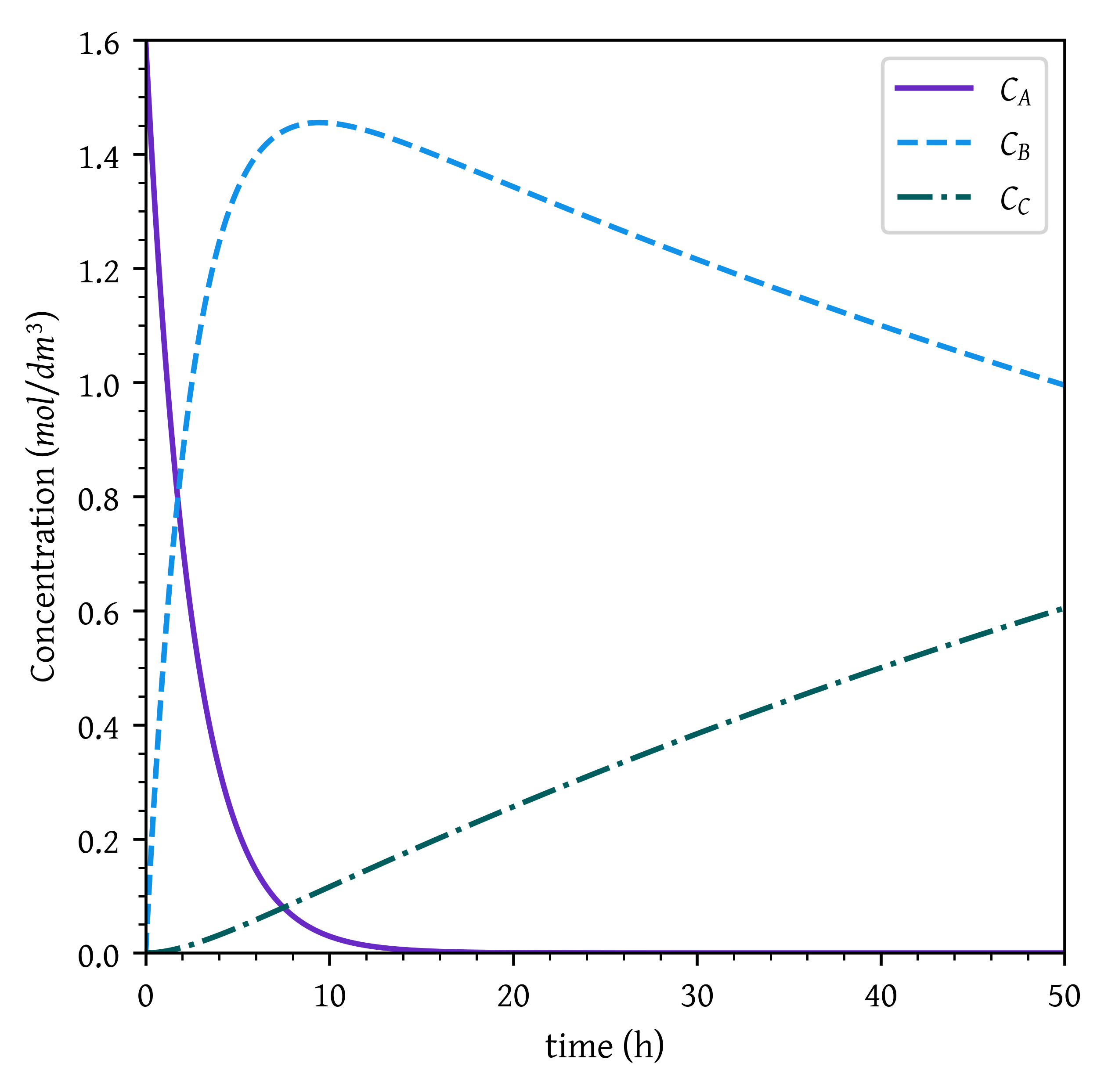
ca, cb, cc = sol.sol(t)plt.plot(t, ca, label='$C_A$')
plt.plot(t, cb, label='$C_B$')
plt.plot(t, cc, label='$C_C$')
plt.xlabel('time (h)')
plt.ylabel('Concentration ($mol/dm^3$)')
# Setting x and y axis limits
plt.xlim(0, t_final)
plt.ylim(0, ca0)
plt.legend()
plt.show()def batch_reactor_b(t, y, *args):
ca, cb, cc = y
k1, k2, km1 = args
r1a = k1 * ca
r1b = km1 * cb
r2b = k2 * cb
dcadt = - r1a + r1b
dcbdt = r1a -r1b - r2b
dccdt = r2b
return [dcadt, dcbdt, dccdt]
km1 = 0.3
# initial conditions
y0 = [ca0, 0, 0]
args = (k1, k2, km1)
t_final = 50
sol = solve_ivp(batch_reactor_b, [0, t_final], y0, args=args, dense_output=True)
t = np.linspace(0,t_final, 1000)
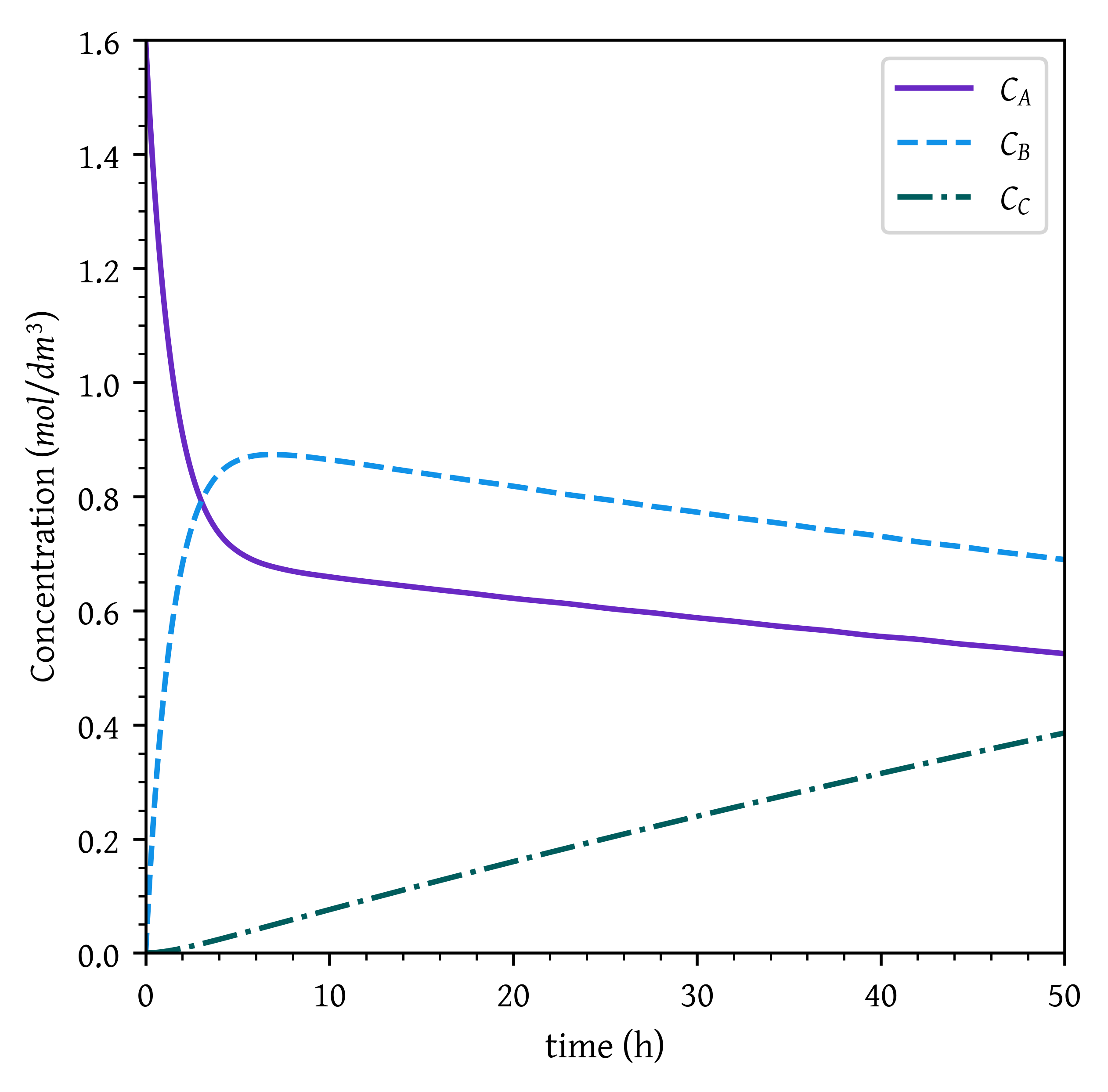
ca, cb, cc = sol.sol(t)plt.plot(t, ca, label='$C_A$')
plt.plot(t, cb, label='$C_B$')
plt.plot(t, cc, label='$C_C$')
plt.xlabel('time (h)')
plt.ylabel('Concentration ($mol/dm^3$)')
# Setting x and y axis limits
plt.xlim(0, t_final)
plt.ylim(0, ca0)
plt.legend()
plt.show()def batch_reactor_c(t, y, *args):
ca, cb, cc = y
k1, k2, km1, km2 = args
r1a = k1 * ca
r1b = km1 * cb
r2b = k2 * cb
r2c = km2 * cc
dcadt = - r1a + r1b
dcbdt = r1a -r1b - r2b + r2c
dccdt = r2b - r2c
return [dcadt, dcbdt, dccdt]
km1 = 0.3
km2 = 0.005
# initial conditions
y0 = [ca0, 0, 0]
args = (k1, k2, km1, km2)
t_final = 50
sol = solve_ivp(batch_reactor_c, [0, t_final], y0, args=args, dense_output=True)
t = np.linspace(0,t_final, 1000)
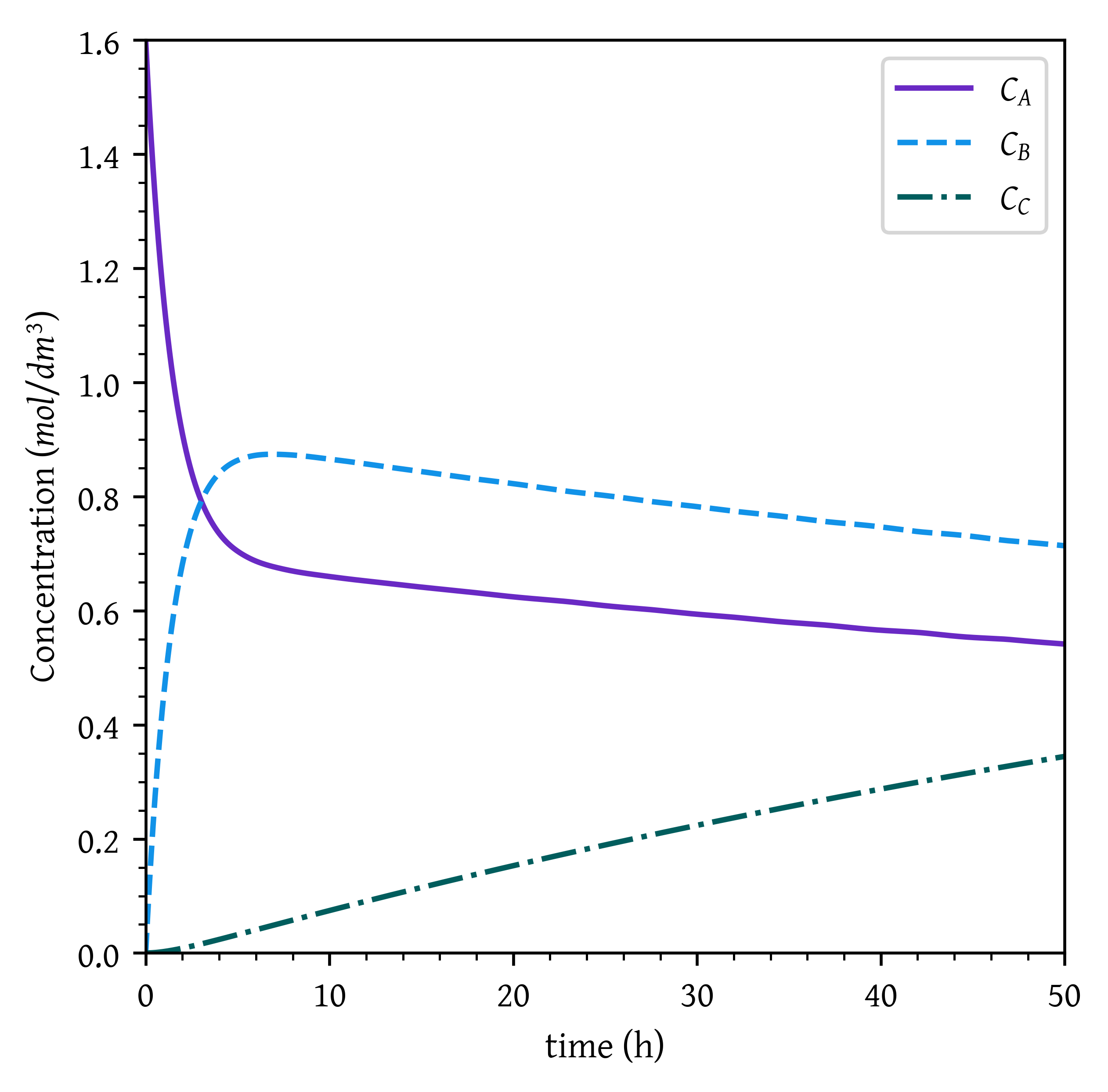
ca, cb, cc = sol.sol(t)plt.plot(t, ca, label='$C_A$')
plt.plot(t, cb, label='$C_B$')
plt.plot(t, cc, label='$C_C$')
plt.xlabel('time (h)')
plt.ylabel('Concentration ($mol/dm^3$)')
# Setting x and y axis limits
plt.xlim(0, t_final)
plt.ylim(0, ca0)
plt.legend()
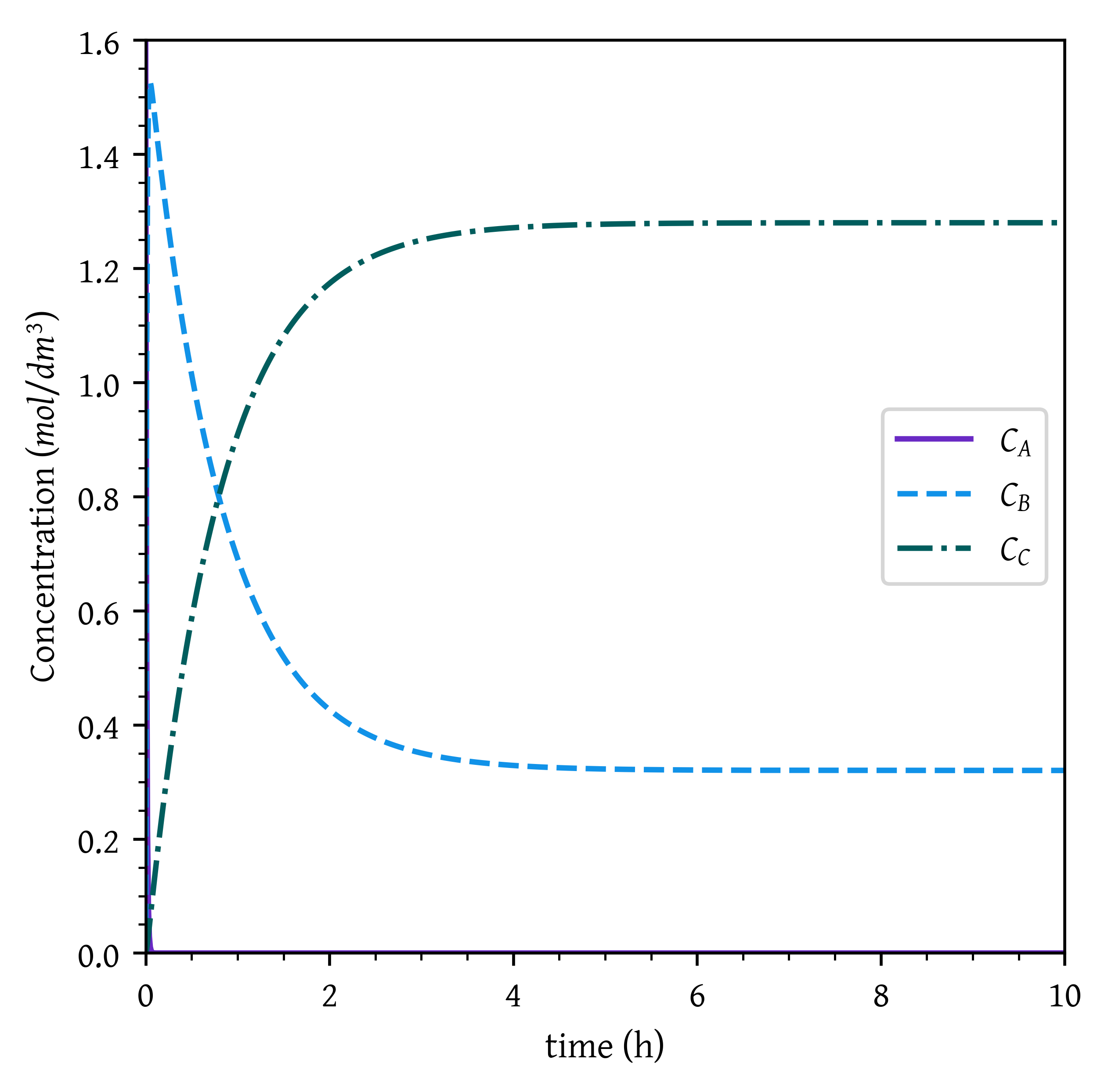
plt.show()k1 = 100
k2 = 1
km1 = 0
km2 = 0
# initial conditions
y0 = [ca0, 0, 0]
args = (k1, k2, km1, km2)
t_final = 10
sol = solve_ivp(batch_reactor_c, [0, t_final], y0, args=args, dense_output=True)
t = np.linspace(0,t_final, 1000)
ca, cb, cc = sol.sol(t)
plt.plot(t, ca, label='$C_A$')
plt.plot(t, cb, label='$C_B$')
plt.plot(t, cc, label='$C_C$')
plt.xlabel('time (h)')
plt.ylabel('Concentration ($mol/dm^3$)')
# Setting x and y axis limits
plt.xlim(0, t_final)
plt.ylim(0, ca0)
plt.legend()
plt.show()When > 100, the first reaction is very fast. Therefore, all A is immediately converted to B and the concentration of B immediately reaches close to .
As < 0.1, the second reaction is much slower. Since both the reactions are irreversible (, and both are zero), B gets slowly converted to C. Given sufficient time, all B is converted to C.
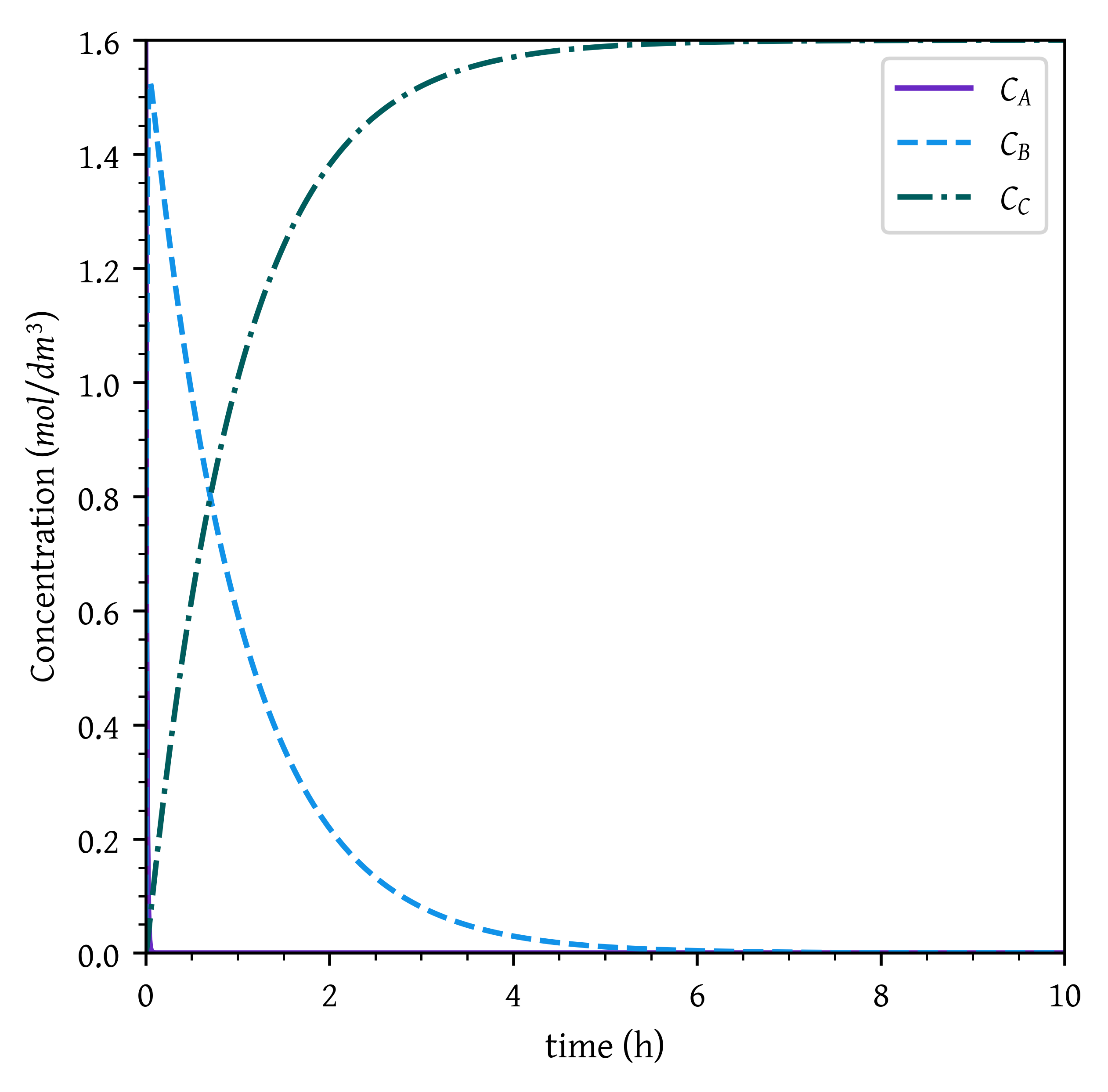
k1 = 100
k2 = 1
km1 = 0
km2 = 1
# initial conditions
y0 = [ca0, 0, 0]
args = (k1, k2, km1, km2)
t_final = 10
sol = solve_ivp(batch_reactor_c, [0, t_final], y0, args=args, dense_output=True)
t = np.linspace(0,t_final, 1000)
ca, cb, cc = sol.sol(t)
plt.plot(t, ca, label='$C_A$')
plt.plot(t, cb, label='$C_B$')
plt.plot(t, cc, label='$C_C$')
plt.xlabel('time (h)')
plt.ylabel('Concentration ($mol/dm^3$)')
# Setting x and y axis limits
plt.xlim(0, t_final)
plt.ylim(0, ca0)
plt.legend()
plt.show()Since second reaction is reversible and , a steady state (equilibrium) is achieved where
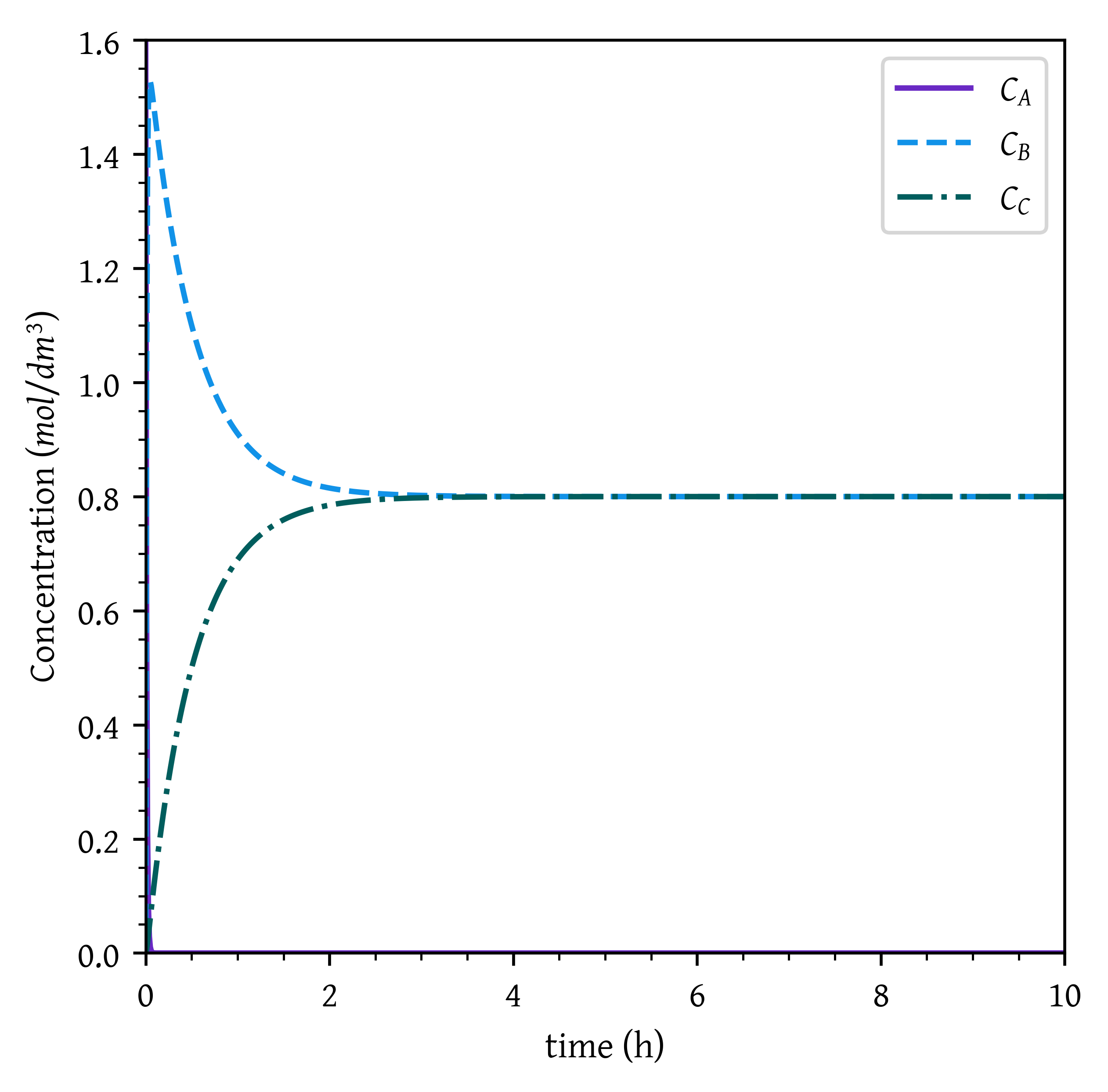
k1 = 100
k2 = 1
km1 = 0
km2 = 0.25
# initial conditions
y0 = [ca0, 0, 0]
args = (k1, k2, km1, km2)
t_final = 10
sol = solve_ivp(batch_reactor_c, [0, t_final], y0, args=args, dense_output=True)
t = np.linspace(0,t_final, 1000)
ca, cb, cc = sol.sol(t)
plt.plot(t, ca, label='$C_A$')
plt.plot(t, cb, label='$C_B$')
plt.plot(t, cc, label='$C_C$')
plt.xlabel('time (h)')
plt.ylabel('Concentration ($mol/dm^3$)')
# Setting x and y axis limits
plt.xlim(0, t_final)
plt.ylim(0, ca0)
plt.legend()
plt.show(), Therefore at equilibrium is achieved where
At equilibrium = 0.32 , = 1.28
References
Citation
@online{utikar2024,
author = {Utikar, Ranjeet},
title = {Solutions to Workshop 06: {Multiple} Reactions},
date = {2024-03-24},
url = {https://cre.smilelab.dev/content/workshops/06-multiple-reactions/solutions.html},
langid = {en}
}