In class activity: Reaction mechanisms and catalysis
Lecture notes for chemical reaction engineering
Gas phase decomposition of azomethane
Consider the gas-phase decomposition of azomethane, AZO, to give ethane and nitrogen
Experimental observations show that the rate of formation of ethane is first order with respect to AZO at pressures greater than 1 atm (relatively high concentrations)
and second order at pressures below 50 mmHg (low concentrations):
We could combine these two observations to postulate a rate law of the form
find a mechanism that is consistent with the experimental observations
Step 1: Propose an active intermediate.
We will choose as an active intermediate an azomethane molecule that has been excited through molecular collisions, to form , that is, .
Step 2: Propose a mechanism
Reaction 1:
Reaction 2:
Reaction 3:
Step 3: Write rate laws
Reaction 1:
Reaction 2:
Reaction 3:
Step 4: Write rate of formation of product.
Step 5: Write net rate of formation of the active intermediate and use the PSSH
Solving for
Step 6: Eliminate the concentration of the active intermediate species in the rate laws by solving the simultaneous equations developed in Steps 4 and 5.
Step 7: Compare with experimental data.
At low AZO concentrations,
for which case we obtain the following second-order rate law:
At high concentrations
in which case the rate expression follows first-order kinetics
Nitric oxide combustion
Find the reaction rate law of the following reaction
Experimental result: At low concentration, the reaction rate decreases with increasing temperature.
Step 1: Propose an active intermediate.
We will choose as an active intermediate .
Step 2: Propose a mechanism
Reaction 1:
Reaction 2:
Reaction 3:
Step 3: Write rate laws
Reaction 1:
Reaction 2:
Reaction 3:
Step 4: Write rate of formation of product.
Step 5: Write net rate of formation of the active intermediate and use the PSSH
Solving for
Step 6: Eliminate the concentration of the active intermediate species in the rate laws by solving the simultaneous equations developed in Steps 4 and 5.
At low NO concentrations,
Step 7: Compare with experimental data.
Experimental result: At low concentration, the reaction rate decreases with increasing temperature.
For reaction rate to decrease with temperature,
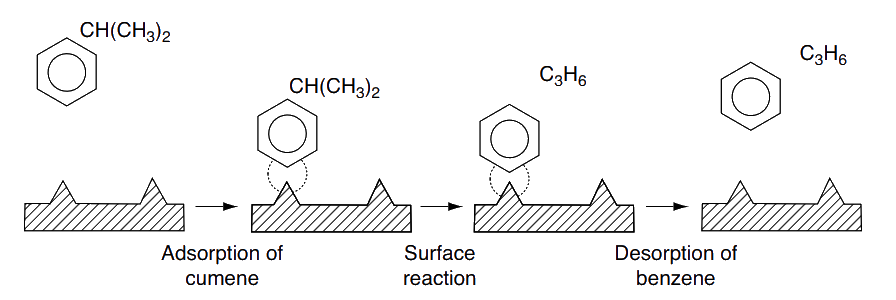
Decomposition of cumene to form benzene and propylene
Develop rate laws for catalytic decomposition of cumene to form benzene and propylene.
Reaction:
Steps:
Adsorption:
Surface reaction:
Desorption:
Reaction rates:
Adsorption:
Surface reaction:
Desorption:
For steady state operations the reaction rates of these three steps are equal.
Case 1: Adsorption of Cumene Rate-Limiting
Because we cannot measure either or , we must replace these variables in the rate law with measurable quantities in order for the equation to be meaningful.
For adsorption-limited reactions, the surface-specific reaction rate , and desorption-specific reaction rate are large by comparison, and we can set
Therefore,
And,
Combining,
and
The concentration of vacant sites, , can now be eliminated by utilizing the site balance to give the total concentration of sites, which is assumed constant.
Solving for we have
and the rate equation
At t = 0 min we have , (no product) and (no product)
Plot initial rate is linear.
Case 2: Surface Reaction Rate-Limiting
- : small
- : large or
- : large or
From Adsorption reaction
From Desorption reaction
From site balance
Therefore,
At t = 0
At low partial pressure of C we have
Initial rate increases linearly with the partial pressure of C
At high initial partial pressure of C we have
Initial rate is constant.
Case 3: Desorption of Benzene the Rate-Limiting
- : small
- : large or
- : large or
From adsorption reaction
From surface reaction
From site balance
Therefore reaction rate:
At t = 0;
Initial rate is constant.
Rate law for catalytic reaction
Find the reaction rate law of the reaction
Reaction:
Mechanism:
Oxygen adsorption (V is vacant site):
Surface reaction:
Reaction rates:
Reaction 1:
Reaction 2:
Assume reaction 2 is slow (rate limiting step)
Therefore,
Rate for reaction 2 then becomes:
Site balance:
Substituting in equation for
at t = 0
Compare with the experimental data
- Differential reactor
- Measureable data
- Keep constant, change
- Keep constant, change
Surface reaction limiting reaction
The reaction is carried out over a solid catalyst, the reaction mechanism is believed to be
Assuming step 3 is the slowest step, derive the rate law for the reaction.
Reaction:
Mechanism:
Adsorption:
Surface reaction:
Desorption:
Reaction rates:
and
Assume surface reaction is rate limiting
We need to eliminate
and
Write site balance to calculate :
Substituting
Initial rates in differential reactor
at t = 0
Citation
@online{utikar2024,
author = {Utikar, Ranjeet},
title = {In Class Activity: {Reaction} Mechanisms and Catalysis},
date = {2024-03-23},
url = {https://cre.smilelab.dev/content/notes/08-reaction-mechanisms-and-catalysis/in-class-activities.html},
langid = {en}
}