In class activity: Conversion and Reactor sizing
Lecture notes for chemical reaction engineering
Design equations in terms of conversion
Derive design equation in terms of conversion for a CSTR
Derive design equation in terms of conversion for a PFR
CSTR sizing
Using the data in Table 1, calculate for , and
PFR sizing
Using the data in Table 2, calculate for , and
An Adiabatic Liquid-Phase Isomerization
The isomerization of butane
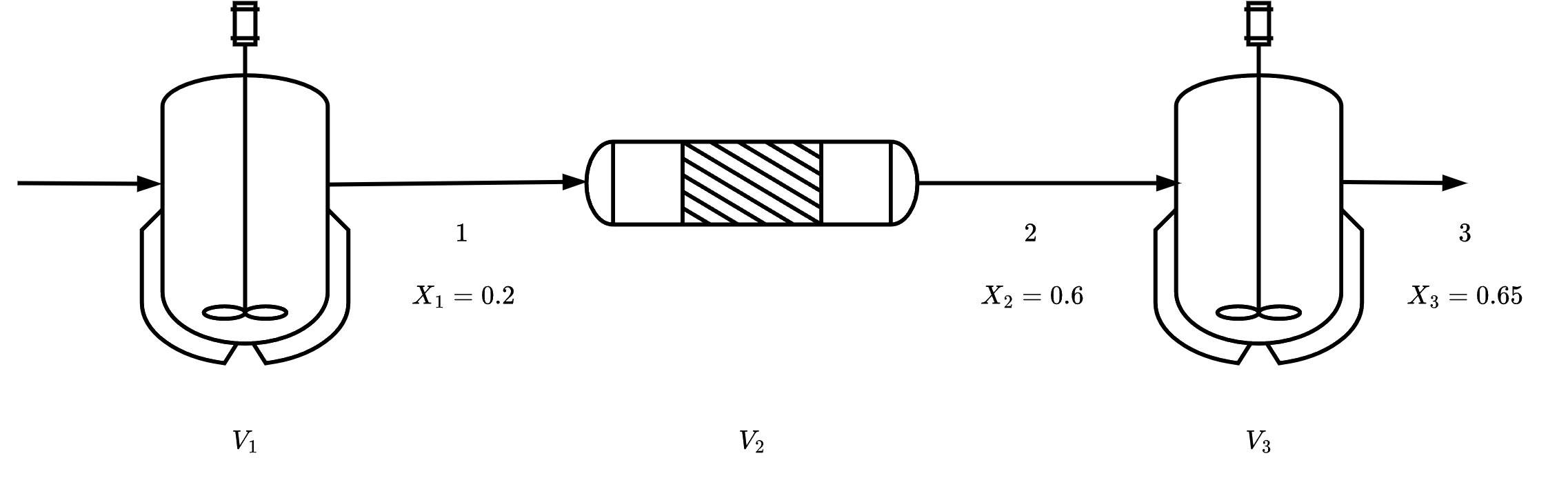
was carried out adiabatically in the liquid phase. The data for this reversible reaction are given in Table 3. The reactor scheme shown below in Figure 1. Calculate the volume of each of the reactors for an entering molar flow rate of n-butane of 50 kmol/hr.
Batch reactor sizing
Discuss how you can use Levenspeil plots to design batch reactors.
We are planning to operate a batch reactor for converting A into R. This is a liquid phase reaction with stoichiometry . How long must we react each batch for concentration to drop from = 1.3 mol/l to = 0.30 mol/l? The data of rate of reaction v/s concentration of A is given in Table 4.
Citation
@online{utikar2024,
author = {Utikar, Ranjeet},
title = {In Class Activity: {Conversion} and {Reactor} Sizing},
date = {2024-03-03},
url = {https://cre.smilelab.dev/content/notes/02-conversion-and-reactor-sizing/in-class-activities.html},
langid = {en}
}