Reaction mechanisms and catalysis
Chemical Reaction Engineering
Nonelementary rate laws
Rate law is typically determined from experimental data
Elementary reaction kinetics: the reaction orders and stoichiometric coefficients are identical
Nonelementary reaction kinetics: no direct correspondence between reaction order and stoichiometry
CHX3CHOCHX4+CO;−rCHX3CHO=kCCHX3CHO3/2
HX2+BrX22HBr;−rHBr=CHX2+k2CBrX2k1CHX2CBrX23/2
Why the rate laws are complicated? → Reaction mechanism
Use the experimental rate law to postulate a reaction mechanism
In practice, knowledge of the reaction mechanism helps use to design better catalyst, troubleshoot, aid in troubleshooting poor reactor performance
Nonelementary rate laws
Nonelementary kinetics are the result of multiple elementary reaction steps
One or more active intermediates are involved
Active Intermediate
A high energy species that reacts further very fast. It is consumed as fast as it is formed
It can be
- A high energy molecule
- A free radical
- An ionic Intermediate
It is present at extremely low concentration.
Pseudo-Steady-State Hypothesis (PSSH)
Net rate of formation of reactive intermediate ≈ 0
If the active intermediate appears in n reactions, then
rA∗=i=1∑nri,A∗=0
How do we determine the mechanism?
Postulate a reaction mechanism that is a series of elementary reactions
Derive a rate equation for the postulated mechanism
Is the rate equation for the postulated mechanism consistent with the experimental results?
Searching for a mechanism
- Propose an active intermediate(s).
- Propose a mechanism, utilizing the rate law obtained from experimental data, if possible.
- Model each reaction in the mechanism sequence as an elementary reaction.
- After writing rate laws for the rate of formation of desired product, write the rate laws for each of the active intermediates.
- Write the net rate of formation for the active intermediate and use the PSSH.
- Eliminate the concentrations of the intermediate species in the rate laws by solving the simultaneous equations developed in Steps 4 and 5.
- If the derived rate law does not agree with experimental observation, assume a new mechanism and/or intermediates and go to Step 3. A strong background in organic and inorganic chemistry is helpful in predicting the activated intermediates for the reaction under consideration.
Rules of thumb for development of a mechanism
Species having the concentration(s) appearing in the denominator of the rate law probably collide with the active intermediate
M+A⋅[Collision products]−rA1∗=k1CMCA⋅
If a constant appears in the denominator, one of the reaction steps is probably the spontaneous decomposition of the active intermediate
A⋅[Decomposition products]−rA2∗=k2CA⋅
Species having the concentration(s) appearing in the numerator of the rate law probably produce the active intermediate in one of the reaction steps
[Reactants]A⋅+[Other products]rA3∗=k3CReactants
PSSH explanation for common first-order reactions
PSSH theory helps to explain why many reactions, such as the decomposition of dimethyl ether commonly exhibit first-order kinetics
(CHX3)X2OCHX4+HX2+CO
AProducts;−rA=kCA
The reaction is first order but not elementary.
Mechanism
Activation:k A+MkX1AX∗+M;r1,AX∗=k1CACM
Deactivation: AX∗+MkX2A+M;r2,AX∗=k2CAX∗CM
Decomposition: AX∗kX3P;r3,AX∗=k3CAX∗
Rate of formation of product
rp=k3CAX∗
Need to eliminate CAX∗
PSSH explanation for common first-order reactions
PSSH: rA∗=0
rA∗k1CACMCA∗rP=k1CACM−k2CA∗CM−k3CA∗=0=CA∗(k2CM+k3)=k2CM+k3k1CACM=k2CM+k3k3k1CACM
CM is constant
k=k2CM+k3k3k1CM
and
−rA=kCA
Catalysts & catalysis
Definition
Fogler: A substance that affects the rate of a reaction but emerges from the process unchanged.
Ostwald (1895): A catalyst accelerates a chemical reaction without affecting the position of the equilibrium.
Positive catalyst increases reaction rate.
Negative catalyst decreases reaction rate.
About 75% of all chemicals are produced by catalytic process.
Catalyst can be:
Gas (NOXx): Nitric Oxide (NO) for the Ostwald Process: In the industrial synthesis of nitric acid, nitric oxide acts as a catalyst in the oxidation of ammonia to nitrogen dioxide. This is part of a gas-phase reaction system where NO is regenerated and acts catalytically.
Liquid (HX2SOX4): Sulfuric Acid in Alkylation: Used in the oil refining industry, sulfuric acid serves as a liquid phase catalyst in alkylation reactions where isobutane is alkylated with olefins to produce higher molecular weight isoparaffins. The reaction occurs in the liquid phase, with sulfuric acid enhancing the reaction by protonating the olefin to form a more reactive carbocation intermediate.
Solid (Ag): Silver on Alumina for Ethylene Oxide Production: Silver deposited on an alumina support is a crucial catalyst for the oxidation of ethylene to ethylene oxide, a precursor in the production of ethylene glycol. This is an example of a solid catalyst used in a gas-phase reaction, highlighting the versatility of catalyst forms and applications.
In this lecture only solid catalysts will be considered.
Heterogeneous catalysis at different scales
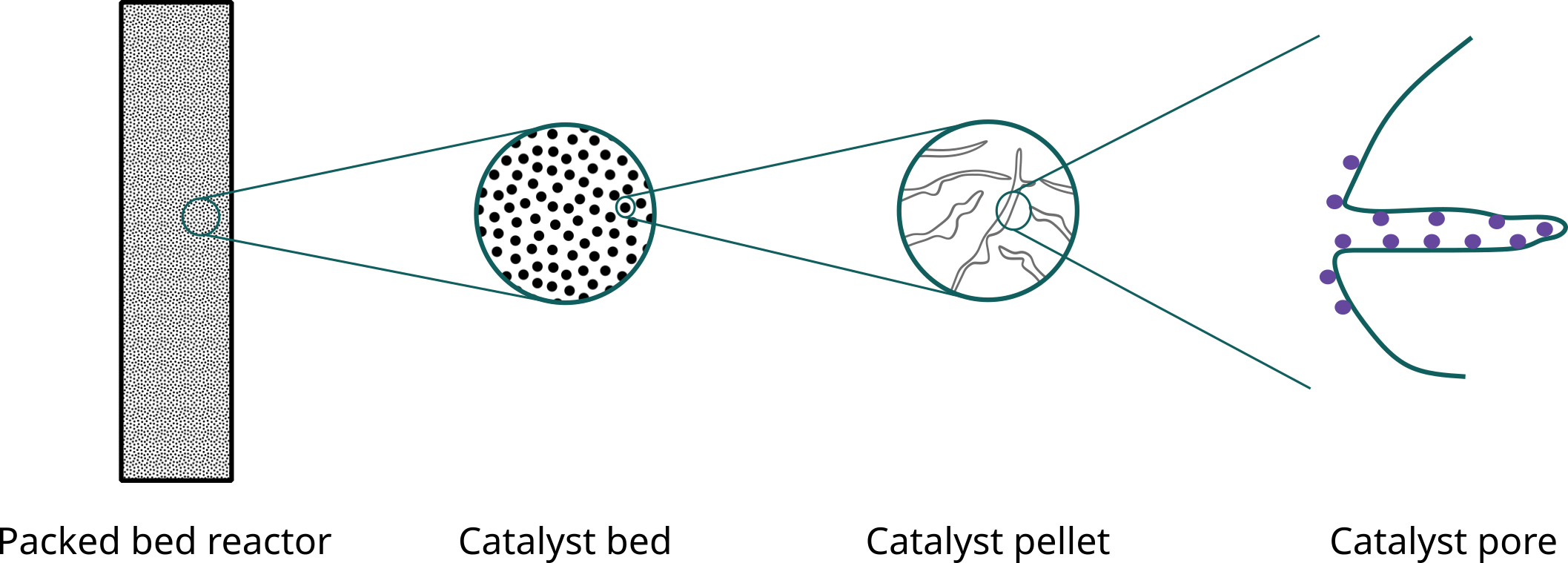
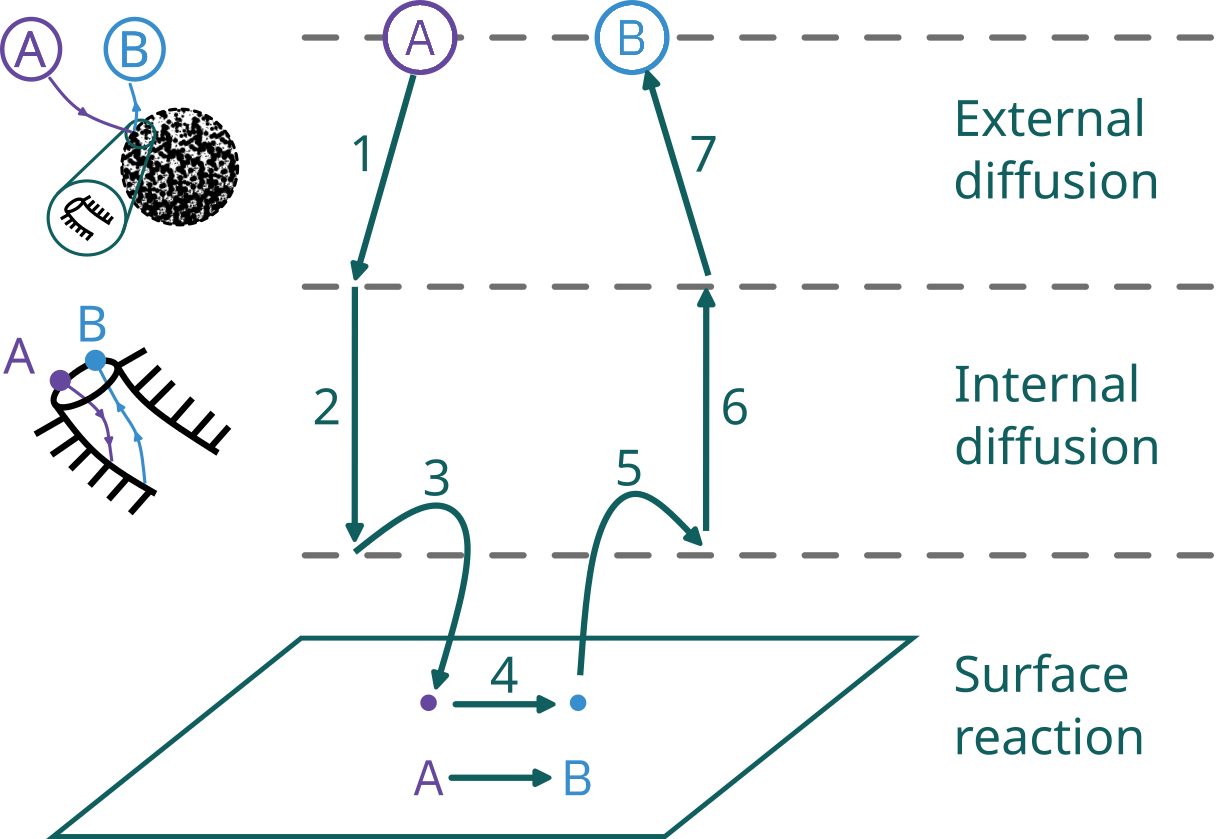
Steps in a heterogeneous catalytic reaction
Adsorption step
Chemisorption is a necessary part of catalytic processes
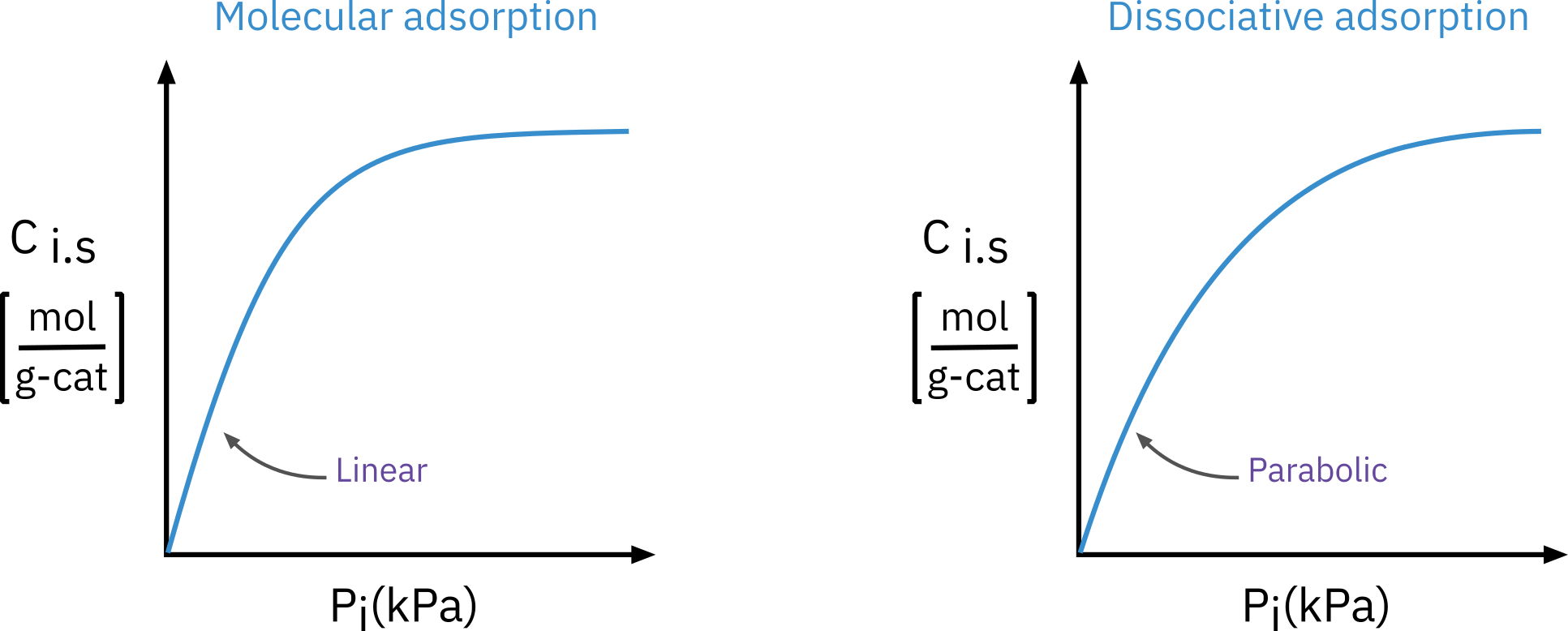
Adsorption data are often presented using adsorption isotherms, which show how much gas is adsorbed by a solid at various pressures at a constant temperature.
We first propose an adsorption mechanism and derive an isotherm from it. This theoretical isotherm is then compared with experimental data.
If the theoretical isotherm aligns well with the experimental data, it suggests that the model accurately reflects the physical processes occurring within the system. Discrepancies between the predicted and actual data indicate that the model may not capture one or more crucial aspects of the physical system.
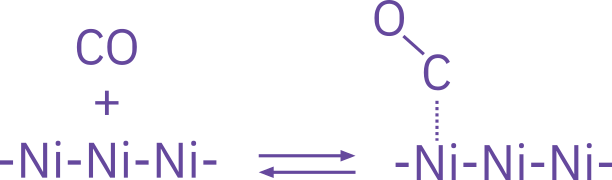
Molecular adsorption
CO+SCO⋅S
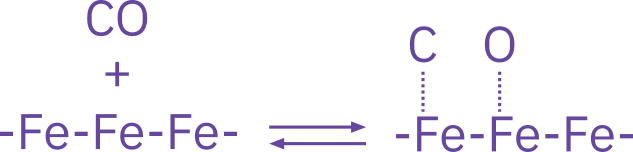
Dissociative adsorption
CO+2SC⋅S+O⋅S
Langmuir isotherm: molecular adsorption
The adsorption of A (gas phase) on an active site S is represented by:
A(g)+SA⋅S
S is a vacant surface site. A⋅S is A bound to a surface site.
Rate of adsorption = rate of attachment - rate of detachment
rAD=kAPACv−k−ACA⋅S
The rate is directly proportional to the number of collisions between the reactant molecules and the catalyst surface, which is influenced by the partial pressure of the reactant (PA).
Additionally, the reaction rate is dependent on the availability of vacant active sites on the catalyst surface.
Adsorption equilibrium constant KA=kA/k−A
rAD=kAPACv−k−ACA⋅SrAD=kA(PACv−kAk−ACA⋅S)
⇒rAD=kA(PACv−KACA⋅S)
Site balance

Ct: Total number of active sites per unit mass of catalyst divided by Avogadro’s number (mol/g cat)
Cv: Number of vacant sites per unit mass of catalyst divided by Avogadro’s number
Cv is not measurable, but the total number of sites Ct can be measured
Site balance
In the absence of catalyst deactivation, assume the total number of active sites remains constant:
Ct=Cv+CA⋅S+CB⋅S
We will use the site balance equation to put Cv in terms of measurable species
Langmuir isotherm: molecular adsorption
Site balance
Ct=Cv+CA⋅S
At equilibrium: The net rate of adsorption is zero. Setting rAD=0 we can obtain equation for concentration of A adsorbed on the surface
CA⋅S=KACvPACA⋅S=KAPA(Ct−CA⋅S)
Langmuir isotherm: By rearranging, we obtain the Langmuir isotherm
CA⋅S=1+KAPAKAPACt
This equation relates the equilibrium concentration of A adsorbed on the surface, CA⋅S, as a function of the partial pressure of A
Linearized form
CA⋅SPA=KACt1+CtPA
Langmuir isotherm: Dissociative adsorption
Consider the adsorption of CO on iron where CO dissociates into separate atoms
CO+2SC⋅S+O⋅S
Dissociative adsorption requires two adjacent vacant sites, unlike molecular adsorption that needs only one.
The likelihood of finding two adjacent vacant sites is proportional to the square of the concentration of vacant sites, in line with the law of mass action.
Consequently, the rate of adsorption is proportional to the product of the carbon monoxide partial pressure and the square of the vacant-site concentration.
For desorption, two adjacent occupied sites are needed, making the rate of desorption proportional to the product of the occupied-site concentrations CC⋅S and CO⋅S.
The net rate of adsorption
rAD=kAPCOCv2−k−ACC⋅SCO⋅S
rAD=kA(PCOCv2−KACC⋅SCO⋅S)KA=k−AkA
Langmuir isotherm: Dissociative adsorption
At equilibrium rAD=0⇒kAPCOCv2=k−ACC⋅SCO⋅S
For CC⋅S=CO⋅S:(KAPCO)1/2Cv=CO⋅S
Substituting for CC⋅S, and CO⋅S in site balance equation
Ct=Cv+CC⋅S+CO⋅S
Ct=Cv+(KAPCO)1/2Cv+(KAPCO)1/2Cv=Cv(1+2(KAPCO)1/2)
Solving for Cv Cv=1+2(KAPCO)1/2Ct
Langmuir isotherm:
CO⋅S=1+2(KAPCO)1/2(KAPCO)1/2Ct;CO⋅S(PCO)1/2=Ct(KA)1/21+Ct2(PCO)1/2
Langmuir isotherm adsorption
Surface reaction step: single site
After a reactant has been adsorbed onto the surface, A⋅S, is capable of reacting in a number of ways to form the reaction product
Single site mechanism
The surface reaction may be a single-site mechanism in which only the site on which the reactant is adsorbed is involved in the reaction.
An adsorbed molecule of A may isomerize (or perhaps decompose) directly on the site to which it is attached, for example, such as pentene isomerization
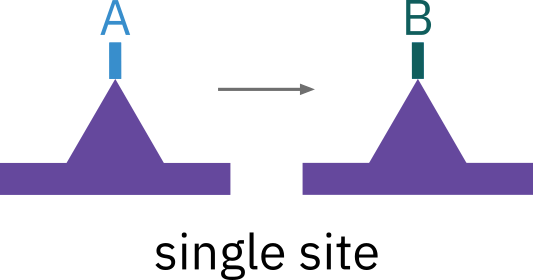
A⋅SB⋅S
- Surface reaction rate law
rS=kSCA⋅S−k−SCB⋅S
rS=kS(CA⋅S−KSCB⋅S)
KS=kS/k−S
Surface reaction step: dual site
- Mechanism 1
A⋅S+SB⋅S+S
rS=kS(CA⋅SCv−KSCB⋅SCv)
- Mechanism 2
A⋅S+B⋅SC⋅S+D⋅S
rS=kS(CA⋅SCB⋅S−KSCC⋅SCD⋅S)
- Mechanism 3: Two different kind of sites are involved
A⋅S+B⋅SX′C⋅SX′+D⋅S
rS=kS(CA⋅SCB⋅SX′−KSCC⋅SX′CD⋅S)
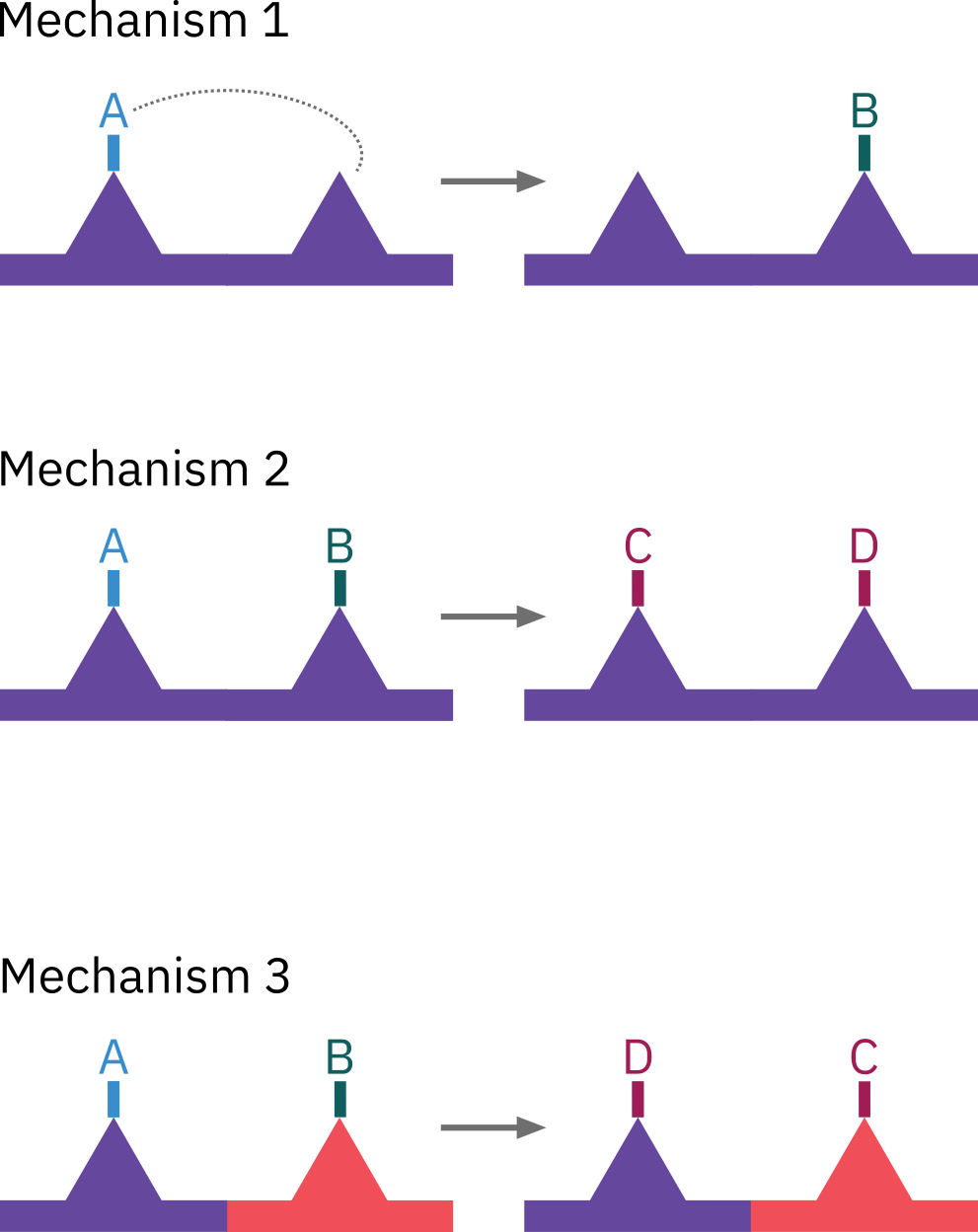
Reactions involving either single- or dual-site mechanisms, are referred to as following Langmuir-Hinshelwood kinetics.
Surface reaction step: Eley-Rideal mechanism
Reaction between adsorbed molecule and a molecule in the gas phase
Unlike the Langmuir-Hinshelwood mechanism it postulates reactions between adsorbed species and molecules directly from the gas phase.
Examples:
- The reaction of propylene and benzene
- Hydrogenation reactions: hydrogen adsorbs on the catalyst surface and reacts with an organic molecule directly striking from the gas phase.
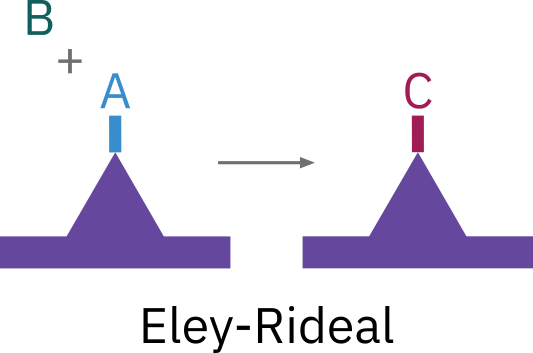
A⋅S+B(g)C⋅S
rS=kS(CA⋅SPB−KSCC⋅S)
Desorption step
Products are desorbed into the gas phase
C⋅SC+S
Rate of desorption
rDC=kD(CC⋅S−KDCPCCv)
The desorption of C is the reverse of the adsorption of C
rDC=−rAD,C
The desorption equilibrium constant KDC is the reciprocal of the adsorption equilibrium constant KC
KDC=KC1
rDC=kD[CC⋅S−KCPCCv]
Rate limiting step
When heterogeneous reactions are carried out at steady state, the rates of each of the three reaction steps in series (adsorption, surface reaction, and desorption) are equal to one another
−rA′=rAD=rS=rD
One step is usually rate-limiting or rate-controlling
- slowest step among all.
- If the rate-limiting step could be sped up, the entire reaction would be faster
- Although reactions involve all 7 steps, if internal and external mass transfer limitations are removed, only adsorption, surface reaction, or desorption will be rate limiting
- Most of the times, the surface reaction step is rate limiting.
- slowest step among all.
Derive a rate law for catalytic reaction
Postulate catalytic mechanism, and then derive the rate law for that mechanism
Assume pseudo-steady state hypothesis (−rA′=rAD=rS=rD)
- No accumulation of species on the surface or near interface
- Each species adsorbed on the surface is a reactive intermediate
- Net rate of formation of species i adsorbed on the surface is 0, ri⋅S=0
Identify rate limiting step
Steps to derive the rate law
- Select among types of adsorption, surface reaction, and desorption
- Write rate laws for each individual step, assuming all are reversible
- Postulate which step is rate limiting
- Use non-rate-limiting steps to eliminate the surface concentration terms that cannot be measured
The approach in determining catalytic and heterogeneous mechanisms is usually termed the Langmuir-Hinshelwood approach, since it is derived from ideas proposed by Hinshelwood based on Langmuir’s principles for adsorption. The Langmuir-Hinshelwood approach was popularized by Hougen and Watson and occasionally includes their names (LHHW kinetics).
Evaluating a catalytic reaction mechanism
- Collect experimental data from test reactor
- Postulate and derive equation for rate law using steps in previous slide
- See if rate law is consistent with data
- If not, then try other surface mechanism (i.e., dual-site adsorption or Eley-Rideal) or choose a different rate-limiting step (adsorption or desorption)
- Repeat until the rate expression describes experimental data
Catalyst decay/ deactivation
Solid catalyst activity changes with operation time.
- Catalyst activity usually decreases with operation time (chemical or/and physical reasons).
- sintering, fouling/ coking, poisoning
Catalyst life time is from few minutes to 10 years.
Regeneration of solid catalyst is important
Effects of catalyst decay on solid catalysed reaction
- Conversion, selectivity decreases
- Require higher reaction temperature
- Pressure drop in a fixed bed reactor increases
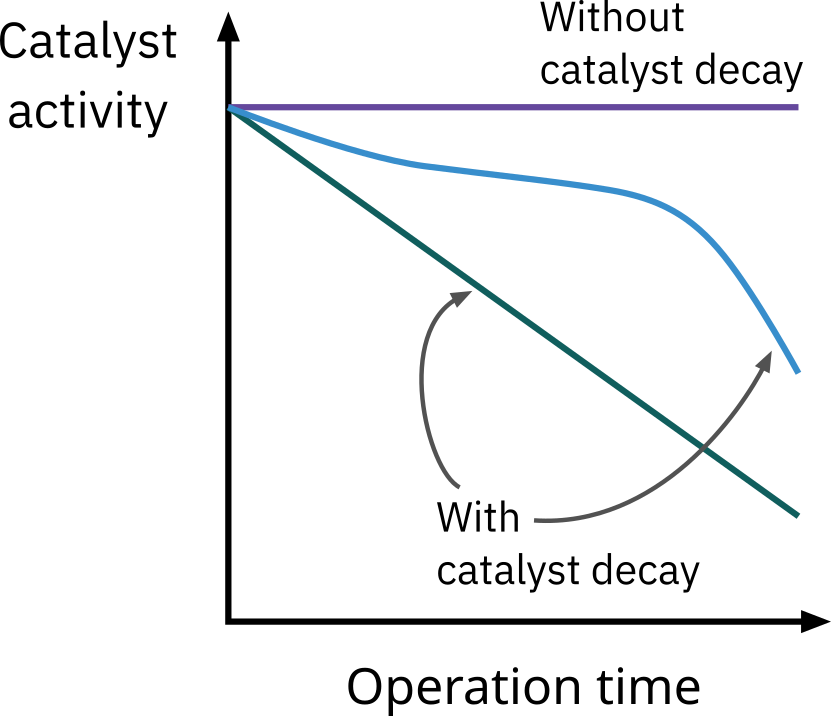
It is very rare to see catalyst activity increases with the operation time
The nickel-based catalysts used in hydroprocessing is often susceptible to sulfur poisoning. However, certain conditioning processes involving controlled exposure to sulfur compounds can rearrange the surface structure, enhancing the catalyst’s activity or selectivity over time.
Sintering
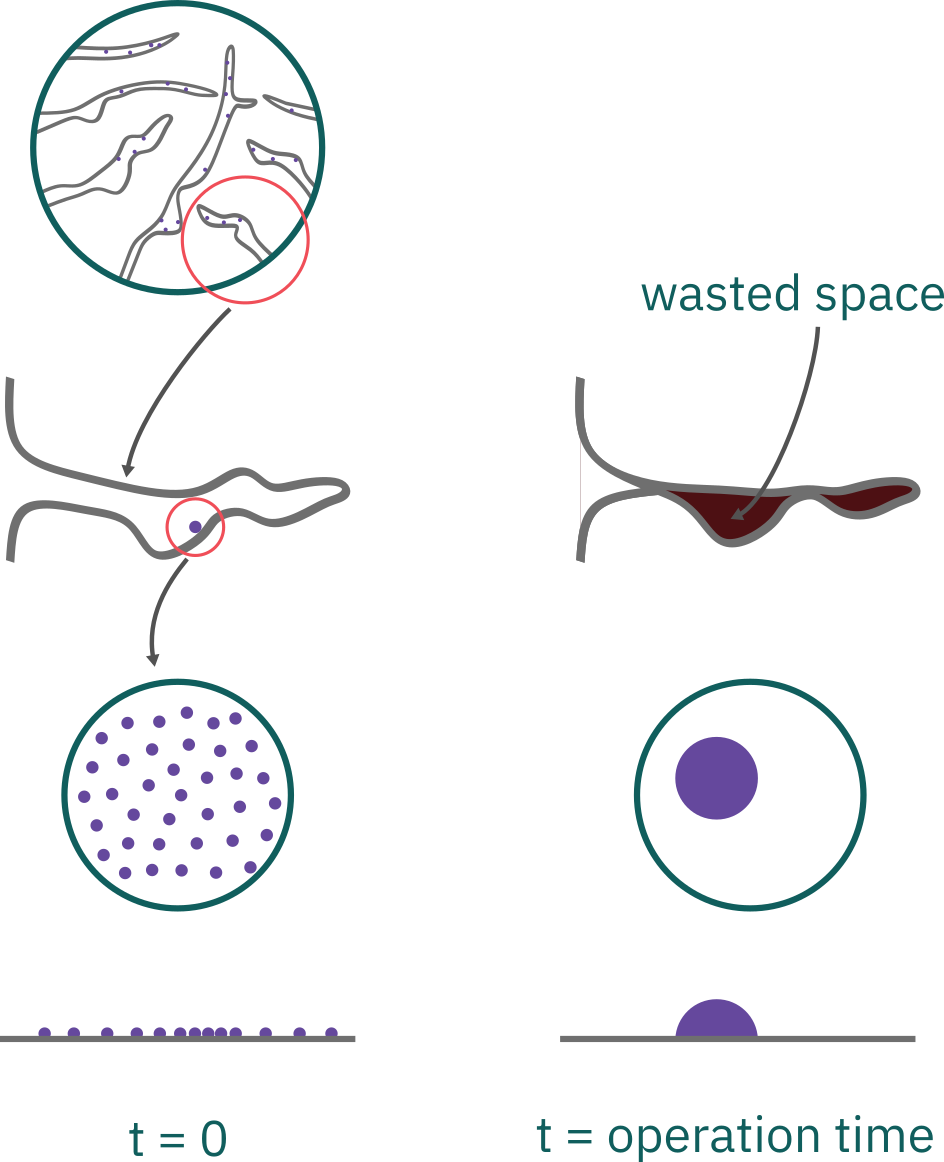
Amount of sintering is usually measured in terms of active surface area of the catalyst Sa
Sa(t)=1+kdtSa0
Loss of catalytic activity due to a loss of active surface area
- growth of the metals deposited on the support
- narrowing or closing of the pores inside the catalyst pellet
Decay law is second order with respect to the present activity
rd=kda2=−dtda
Integrating with a=1 at time t=0 for constant kd
a(t)=1+kdt1
The sintering decay constant follows Arrhenius equation
kd=kd(T0)exp[REd(T01−T1)]
Fouling/ Coking
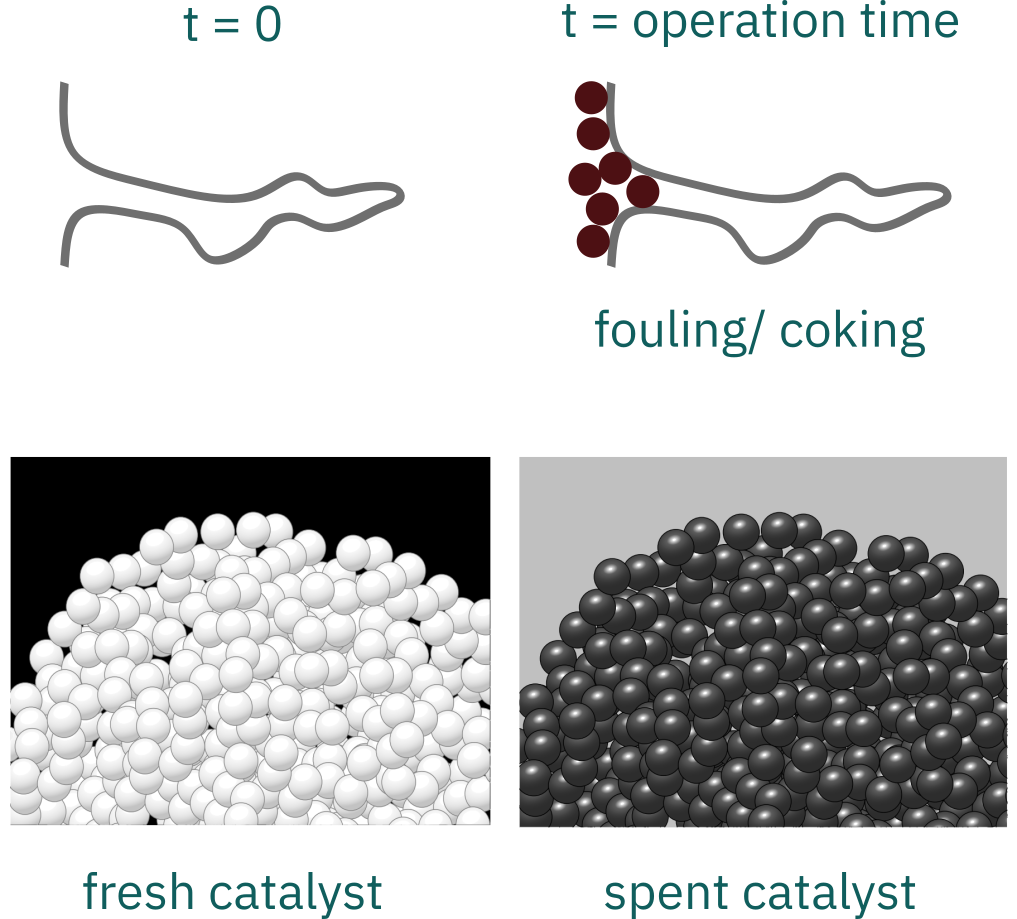
When possible, coking can be reduced by running at elevated pressures (2000–3000 kPa) and hydrogen-rich streams.
Catalysts deactivated by coking can usually be regenerated by burning off the carbon.
Results of carbonaceous (coke) material deposited on the surface of a catalyst
This mechanism is common to reactions involving hydrocarbons.
The amount of coke on the surface after a time t
CC=Atn
For cracking of crude oil in fixed bed
CC=0.52t0.38
Empirical relationships between a and CC
aaa===kCkCCp+11e−α1CC1+α2CC1
Poisoning
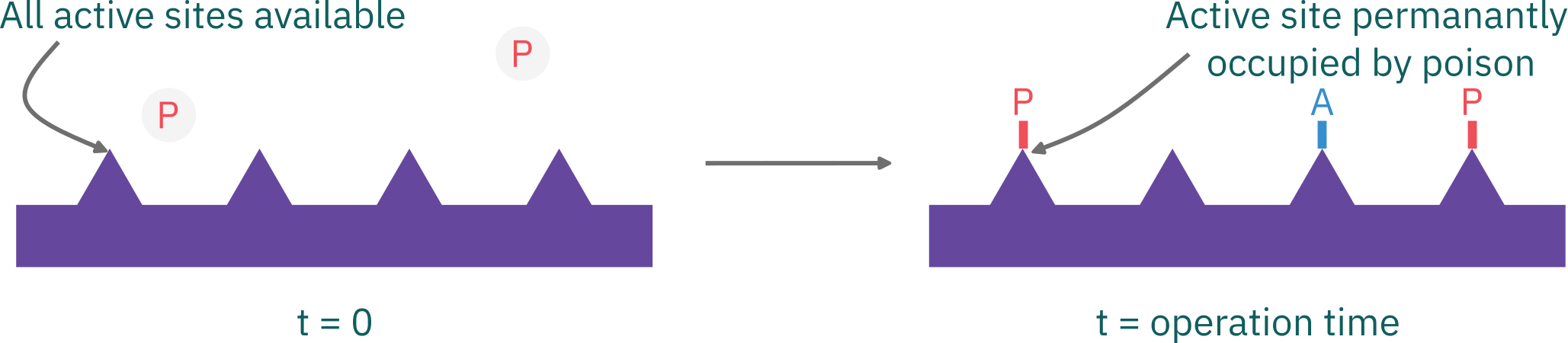
- The poisoning molecules become irreversibly chemisorbed to active sites
- Reduces the number of sites available for the main reaction.
- The poisoning molecule, P, may be a reactant and/or a product in the main reaction, or it may be an impurity in the feed stream.
Main reaction:⎩⎨⎧A+SA⋅SA⋅SB⋅S+C(g)B⋅SB+S⎭⎬⎫−rA′=a(t)1+KACA+KBCBkACA
Poisoning reaction:P+SP⋅S−rd′=−dtda=kd′Cpmaq
Poisoning
Rate of depletion of the poison from the reactant gas stream onto the catalyst sites, rP⋅S, is proportional to the number of sites that are unpoisoned (Ct0−CP⋅S) and the concentration of poison in the gas phase is CP
rP⋅S=kd(Ct0−CP⋅S)CP
This rate is also equal to the rate of removal of total active sites (Ct) from the surface
−dtdCt=dtdCP⋅S=rP⋅S=kd(Ct0−CP⋅S)CP
Dividing through by Ct0 dtdf=kd(1−f)CPf:fraction of sites that have been poisoned
The fraction of sites available for adsorption (1−f) is the activity a(t)
−dtda=a(t)kdCP
Decay rate laws
| Functional Form of Activity | Decay Reaction Order | Differential Form | Integral Form | Examples |
|---|---|---|---|---|
| Linear | 0 | −da/dt=β0 | a=1−β0t | Conversion of para-hydrogen on tungsten when poisoned with O2 |
| Exponential | 1 | −da/dt=β1a | a=e−β1t | Ethylene hydrogenation on Cu poisoned with CO; Cracking of gas oil; Vinyl chloride monomer formation |
| Hyperbolic | 2 | −da/dt=β2a2 | 1/a=1+β2t | Cyclohexane dehydrogenation on Pt/Al2O3; Isobutylene hydrogenation on Ni |
| Reciprocal power | - | −da/dt=β3a1/5 | a=A0t−β3 | Cracking of gas oil and gasoline on clay |
Catalyst regenration

This component is an instance of the CodeMirror interactive text editor. The editor has been configured so that the Tab key controls the indentation of code. To move focus away from the editor, press the Escape key, and then press the Tab key directly after it. Escape and then Shift-Tab can also be used to move focus backwards.

Chemical Reaction Engineering