Non-isothermal reactor design
Chemical Reaction Engineering
Need for energy balance
Consider a liquid phase PFR for a first-order exothermic reaction.
Mole balance
d X A d V = − r A F A 0
\frac{dX_A}{dV} = \frac{-r_A}{F_{A0}}
d V d X A = F A 0 − r A
Rate law
− r A = k C A = − k 0 exp [ E R ( 1 T 1 − 1 T ) ] C A
-r_A = kC_A = -k_0 \exp \left[ \frac{E}{R} \left( \frac{1}{T_1} - \frac{1}{T} \right)\right] C_A
− r A = k C A = − k 0 exp [ R E ( T 1 1 − T 1 ) ] C A
Stoichiometry
C A = C A 0 ( 1 − X A )
C_A = C_{A0}(1-X_A)
C A = C A 0 ( 1 − X A )
Combine: Put F A 0 F_{A0} F A 0 C A 0 C_{A0} C A 0
d X d V = − k 0 exp [ E R ( 1 T 1 − 1 T ) ] ( 1 − X A ) υ 0
\frac{dX}{dV} =
-k_0 \exp \left[ \frac{E}{R} \left( \frac{1}{T_1} - \frac{1}{T} \right)\right]
\frac{(1 - X_A)}{\upsilon_0}
d V d X = − k 0 exp [ R E ( T 1 1 − T 1 ) ] υ 0 ( 1 − X A )
Evaluate
We cannot solve this equation because both X X X T T T V V V X X X V V V T T T
⇒ \Rightarrow ⇒
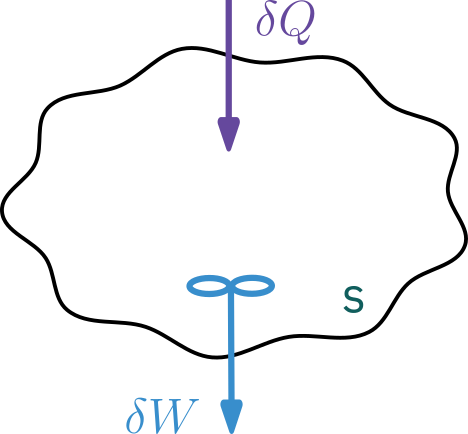
Thermodynamics in a closed system
First law of thermodynamics
System
A system is any bounded portion of the universe, moving or stationary, which is chosen for the application of the various thermodynamic equations.
Closed system: no mass crosses boundaries
Change in Heat flow Work done by total energy = to − by the system of the system the system on the surroundings
\begin{aligned}
\text{Change in}
& & \text{Heat flow}
& & \text{Work done by}
\\
\text{total energy}
&\;\; = & \text{to} \qquad
&\;\; - & \text{by the system}
\\
\text{of the system}
& & \text{the system}
& & \text{on the surroundings}
\end{aligned}
Change in total energy of the system = Heat flow to the system − Work done by by the system on the surroundings
d E ^ = δ Q − δ W
d \hat{E} = \delta Q - \delta W
d E ^ = δ Q − δ W
E ^ \hat{E} E ^ δ Q \delta Q δ Q δ \delta δ
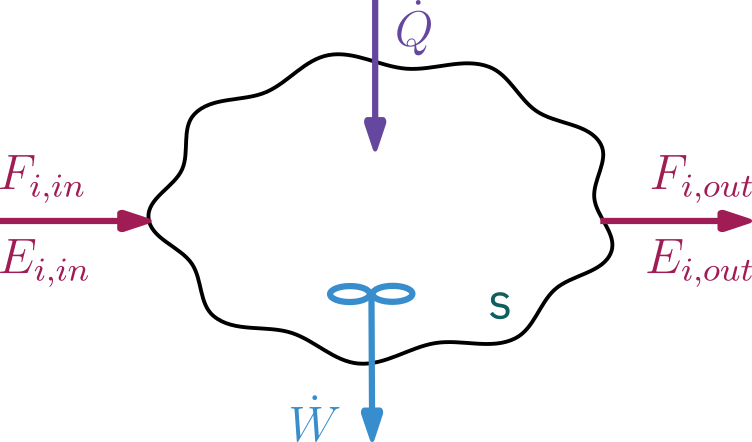
Thermodynamics in an open system
Open system: continuous flow system, mass crosses the system’s boundaries
Mass flow can add or remove energy
System volume is well mixed
N N N F i F_i F i E i E_i E i
Rate of accumulation Work done Energy added Energy leaving of energy = Heat in − by + to the system − the system by in the system the system by mass flow in mass flow out
\begin{aligned}
\text{Rate of accumulation}
& &
& & \text{Work done}
& & \text{Energy added}
& & \text{Energy leaving}
\\
\text{of energy}
&\;\; = & \text{Heat in}
&\;\; - & \text{by} \qquad
&\;\; + & \text{to the system}
&\;\; - & \text{the system by}
\\
\text{in the system}
& &
& & \text{the system}
& & \text{by mass flow in}
& & \text{mass flow out}
\end{aligned}
Rate of accumulation of energy in the system = Heat in − Work done by the system + Energy added to the system by mass flow in − Energy leaving the system by mass flow out
d E ^ s y s d t = Q ˙ − W ˙ + ∑ i = 1 N E i F i ∣ i n − ∑ i = 1 N E i F i ∣ o u t
\frac{d \hat{E}_{sys}}{dt} =
\dot Q
- \dot W
+ \left. \sum_{i=1}^{N} E_i F_i \right|_{in}
- \left. \sum_{i=1}^{N} E_i F_i \right|_{out}
d t d E ^ sy s = Q ˙ − W ˙ + i = 1 ∑ N E i F i in − i = 1 ∑ N E i F i o u t
The work term, W ˙ \dot W W ˙
W ˙ \dot W W ˙
Flow work: work required to get the mass into and out of system
Other work (shaft work, W ˙ s \dot W_s W ˙ s
W ˙ = − ∑ i = 1 n F i P V ~ i ∣ i n + ∑ i = 1 n F i P V ~ i ∣ o u t ⏞ flow work + W ˙ s ⏟ shaft work
\dot{W} =\overbrace{
-\left.\sum_{i=1}^n F_i P \tilde V_i \right|_{in}
+\left.\sum_{i=1}^n F_i P \tilde V_i \right|_{out}}^{\color{RoyalBlue}{\text{flow work}}}
+\underbrace{\dot W_s}_{\color{RoyalBlue}{\text{shaft work}}}
W ˙ = − i = 1 ∑ n F i P V ~ i in + i = 1 ∑ n F i P V ~ i o u t flow work + shaft work W ˙ s
P P P V ~ i \tilde V_i V ~ i i i i
In most instances, the flow-work term is combined with those terms in the energy balance that represent the energy exchange by mass flow across the system boundaries.
d E ^ s y s d t = Q ˙ − W ˙ s + ∑ i = 1 N F i ( E i + P V ~ i ) ∣ i n − ∑ i = 1 N F i ( E i + P V ~ i ) ∣ o u t
\frac{d \hat{E}_{sys}}{dt} =
\dot Q
- \dot W_s
+ \left. \sum_{i=1}^{N} F_i (E_i + P \tilde V_i) \right|_{in}
- \left. \sum_{i=1}^{N} F_i (E_i + P \tilde V_i) \right|_{out}
d t d E ^ sy s = Q ˙ − W ˙ s + i = 1 ∑ N F i ( E i + P V ~ i ) in − i = 1 ∑ N F i ( E i + P V ~ i ) o u t
The energy term, E i E_i E i
The energy E i E_i E i
E i = U i + u i 2 2 + g z i + other
E_i = U_i + \frac {u_i^2} {2} + g z_i + \text{other}
E i = U i + 2 u i 2 + g z i + other
Generally in chemical reactors, U i ≫ u i 2 / 2 + g z i + other U_i \gg {u_i^2}/{2} + g z_i + \text{other} U i ≫ u i 2 / 2 + g z i + other
E i ≈ U i E_i \approx U_i E i ≈ U i
d E ^ s y s d t = Q ˙ − W ˙ s + ∑ i = 1 N F i ( U i + P V ~ i ) ∣ i n − ∑ i = 1 N F i ( U i + P V ~ i ) ∣ o u t
\frac{d \hat{E}_{sys}}{dt} =
\dot Q
- \dot W_s
+ \left. \sum_{i=1}^{N} F_i (U_i + P \tilde V_i) \right|_{in}
- \left. \sum_{i=1}^{N} F_i (U_i + P \tilde V_i) \right|_{out}
d t d E ^ sy s = Q ˙ − W ˙ s + i = 1 ∑ N F i ( U i + P V ~ i ) in − i = 1 ∑ N F i ( U i + P V ~ i ) o u t
Enthalpy H i H_i H i H i = U i + P V ~ i H_i = U_i + P \tilde V_i H i = U i + P V ~ i
d E ^ s y s d t = Q ˙ − W ˙ s + ∑ i = 1 N F i 0 H i 0 − ∑ i = 1 N F i H i
\frac{d \hat{E}_{sys}}{dt} =
\dot Q
- \dot W_s
+ \sum_{i=1}^{N} F_{i0} H_{i0}
- \sum_{i=1}^{N} F_{i} H_{i}
d t d E ^ sy s = Q ˙ − W ˙ s + i = 1 ∑ N F i 0 H i 0 − i = 1 ∑ N F i H i
Steady state energy balance: conversion
Consider reaction
a A + b B → c C + d D \ce{aA + bB -> cC + dD} a A + b B c C + d D
A + b a B → c a C + d a D \ce{A + \frac{b}{a} B -> \frac{c}{a} C + \frac{d}{a} D} A + a b B a c C + a d D
F i = F A 0 ( Θ i + ν i X ) F_i = F_{A0}(\Theta_i + \nu_i X) F i = F A 0 ( Θ i + ν i X )
Θ i = F i 0 F A 0 \Theta_i = \frac{F_{i0}}{F_{A0}} Θ i = F A 0 F i 0
ν i \nu_i ν i
F A = F A 0 ( 1 − X ) F_A = F_{A0}(1 - X) F A = F A 0 ( 1 − X )
F B = F A 0 ( Θ B − b a X ) F_B = F_{A0}(\Theta_B - \frac{b}{a} X) F B = F A 0 ( Θ B − a b X )
F C = F A 0 ( Θ C + c a X ) F_C = F_{A0}(\Theta_C + \frac{c}{a} X) F C = F A 0 ( Θ C + a c X )
F D = F A 0 ( Θ D + d a X ) F_D = F_{A0}(\Theta_D + \frac{d}{a} X) F D = F A 0 ( Θ D + a d X )
F I = F A 0 ( Θ I ) F_I = F_{A0}(\Theta_I) F I = F A 0 ( Θ I )
Q ˙ − W ˙ s + ∑ i = 1 N F i 0 H i 0 − ∑ i = 1 N F i H i = 0
\dot Q
- \dot W_s
+ \sum_{i=1}^{N} F_{i0} H_{i0}
- \sum_{i=1}^{N} F_{i} H_{i}
= 0
Q ˙ − W ˙ s + i = 1 ∑ N F i 0 H i 0 − i = 1 ∑ N F i H i = 0
Steady state energy balance: conversion
∑ i = 1 N F i 0 H i 0 − ∑ i = 1 N F i H i = F A 0 [ ( H A 0 − H A ) + ( H B 0 − H B ) Θ B + ( H C 0 − H C ) Θ C + ( H D 0 − H D ) Θ D + ( H I 0 − H I ) Θ I ] − [ d a H D + c a H C − b a H B − H A ] ⏟ Δ H R x at temperature T F A 0 X = F A 0 ∑ i = 1 N ( H i 0 − H i ) − Δ H R x F A 0 X
\begin{aligned}
\sum_{i=1}^{N} F_{i0} H_{i0} - \sum_{i=1}^{N} F_{i} H_{i}
= & \;\; F_{A0}\left[
(H_{A0} - H_A)
+ (H_{B0} - H_B) \Theta_B
\right. \\
& \left. + (H_{C0} - H_C) \Theta_C
+ (H_{D0} - H_D) \Theta_D
+ (H_{I0} - H_I) \Theta_I
\right] \\
&\;\; - \underbrace{
\color{RoyalBlue}{
\left[
\frac{d}{a} H_D
+ \frac{c}{a} H_C
- \frac{b}{a} H_B
- H_A
\right]
}}_{\Delta H_{Rx} \text{at temperature T}} F_{A0} X \\
= &\;\;
F_{A0} \sum_{i=1}^{N} (H_{i0} - H_i)
- \Delta H_{Rx} F_{A0} X
\end{aligned}
i = 1 ∑ N F i 0 H i 0 − i = 1 ∑ N F i H i = = F A 0 [ ( H A 0 − H A ) + ( H B 0 − H B ) Θ B + ( H C 0 − H C ) Θ C + ( H D 0 − H D ) Θ D + ( H I 0 − H I ) Θ I ] − Δ H R x at temperature T [ a d H D + a c H C − a b H B − H A ] F A 0 X F A 0 i = 1 ∑ N ( H i 0 − H i ) − Δ H R x F A 0 X
Q ˙ − W ˙ s + F A 0 ∑ i = 1 N Θ i ( H i 0 − H i ) − Δ H R x F A 0 X = 0
\dot Q
- \dot W_s
+ F_{A0} \sum_{i=1}^{N} \Theta_i (H_{i0} - H_i)
- \Delta H_{Rx} F_{A0} X
= 0
Q ˙ − W ˙ s + F A 0 i = 1 ∑ N Θ i ( H i 0 − H i ) − Δ H R x F A 0 X = 0
Use this equation when enthalpies are available.
If there is a phase change, we must use this equation for energy balance.
In most cases, the work done W ˙ s \dot W_s W ˙ s
Specific enthalpy H i H_i H i
Specific enthalpy of species i i i
H i = H i ∘ ( T R ) + Δ H Q i H_i = H_i^\circ(T_R) + \Delta H_{Qi} H i = H i ∘ ( T R ) + Δ H Q i
Ignoring enthalpy of mixing
H i ∘ ( T R ) H_i^\circ(T_R) H i ∘ ( T R ) i i i T R T_R T R
Δ H Q i \Delta H_{Qi} Δ H Q i T R T_R T R T T T
In absence of phase change, Δ H Q i = ∫ T 1 T 2 C P i d T \Delta H_{Qi} = \int_{T_1}^{T_2} C_{P_i} dT Δ H Q i = ∫ T 1 T 2 C P i d T
The heat capacity at temperature T T T
C P i = α i + β i T + γ i T 2 C_{P_i} = \alpha_i + \beta_i T + \gamma_i T^2 C P i = α i + β i T + γ i T 2
H i = H i ∘ ( T R ) + ∫ T 1 T 2 C P i d T H_i = H_i^\circ(T_R) + \int_{T_1}^{T_2} C_{P_i} dT H i = H i ∘ ( T R ) + ∫ T 1 T 2 C P i d T
Steady state energy balance
Constant heat capacity
Consider constant heat capacity: \qquad ∫ T i 0 T C P i d T = C P i [ T − T i 0 ] \int_{T_{i0}}^{T} C_{P_i} dT = C_{P_i} \left[ T - T_{i0}\right] ∫ T i 0 T C P i d T = C P i [ T − T i 0 ]
H i 0 = H i ∘ ( T R ) + C P i [ T i 0 − T R ] H_{i0} = H_i^\circ(T_R) + C_{P_i} \left[ T_{i0} - T_{R} \right] H i 0 = H i ∘ ( T R ) + C P i [ T i 0 − T R ]
H i = H i ∘ ( T R ) + C P i [ T i − T R ] H_{i} = H_i^\circ(T_R) + C_{P_i} \left[ T_{i} - T_{R} \right] H i = H i ∘ ( T R ) + C P i [ T i − T R ]
H i 0 − H i = ( H i ∘ ( T R ) + C P i [ T i 0 − T R ] ) − ( H i ∘ ( T R ) + C P i [ T i − T R ] )
H_{i0} - H_i
= \left( \cancel{H_i^\circ(T_R)} + C_{P_i} \left[ T_{i0} - \cancel{T_{R}}\right] \right)
- \left( \cancel{H_i^\circ(T_R)} + C_{P_i} \left[ T_{i} - \cancel{T_{R}}\right] \right)
H i 0 − H i = ( H i ∘ ( T R ) + C P i [ T i 0 − T R ] ) − ( H i ∘ ( T R ) + C P i [ T i − T R ] )
H i 0 − H i = C P i [ T i 0 − T i ] = − C P i [ T i − T i 0 ]
H_{i0} - H_i = C_{P_i} \left[ T_{i0} - T_{i}\right]
= - C_{P_i} \left[ T_{i} - T_{i0}\right]
H i 0 − H i = C P i [ T i 0 − T i ] = − C P i [ T i − T i 0 ]
Q ˙ − W ˙ s − F A 0 ∑ i = 1 N Θ i C P i [ T i − T i 0 ] − Δ H R x F A 0 X = 0
\dot Q
- \dot W_s
- F_{A0} \sum_{i=1}^{N} \Theta_i C_{P_i} \left[ T_{i} - T_{i0}\right]
- \Delta H_{Rx} F_{A0} X
= 0
Q ˙ − W ˙ s − F A 0 i = 1 ∑ N Θ i C P i [ T i − T i 0 ] − Δ H R x F A 0 X = 0
Heat of reaction, Δ H R x ( T ) \Delta H_{Rx}(T) Δ H R x ( T )
Δ H R x ( T ) = d a H D ( T ) + c a H C ( T ) − b a H B ( T ) − H A ( T ) \Delta H_{Rx}(T) =
\frac{d}{a} H_D (T)
+ \frac{c}{a} H_C (T)
- \frac{b}{a} H_B (T)
- H_A (T) Δ H R x ( T ) = a d H D ( T ) + a c H C ( T ) − a b H B ( T ) − H A ( T )
Specific enthalpy of each species
H i = C P i [ T − T R ] H_i = C_{P_i} \left[ T - T_{R}\right] H i = C P i [ T − T R ]
Δ H R x ( T ) = [ d a H D ∘ ( T R ) + c a H C ∘ ( T R ) − b a H B ∘ ( T R ) − H A ∘ ( T R ) ⏟ Δ H R x ∘ at T R ] + [ d a C P D + c a C P C − b a C P B − C P A ⏟ Δ C P ] ( T − T R )
\begin{aligned}
\Delta H_{Rx}(T) = & &
\left[
\underbrace{
\frac{d}{a} H_D^\circ (T_R)
+ \frac{c}{a} H_C^\circ (T_R)
- \frac{b}{a} H_B^\circ (T_R)
- H_A^\circ (T_R)
}_{\color{RoyalBlue}{\Delta H_{Rx}^\circ \text{ at } T_R}}
\right]
& + & \left[
\underbrace{
\frac{d}{a} C_{P_D}
+ \frac{c}{a} C_{P_C}
- \frac{b}{a} C_{P_B}
- C_{P_A}
}_{\color{RoyalBlue}{\Delta C_{P}}}
\right] \left(T - T_R \right)
\end{aligned}
Δ H R x ( T ) = Δ H R x ∘ at T R a d H D ∘ ( T R ) + a c H C ∘ ( T R ) − a b H B ∘ ( T R ) − H A ∘ ( T R ) + Δ C P a d C P D + a c C P C − a b C P B − C P A ( T − T R )
Δ H R x ( T ) = Δ H R x ∘ ( T R ) + Δ C P ( T − T R )
\Delta H_{Rx}(T) = \Delta H_{Rx}^\circ(T_R) + \Delta C_{P}(T - T_R)
Δ H R x ( T ) = Δ H R x ∘ ( T R ) + Δ C P ( T − T R )
Steady state energy balance
Constant heat capacity, adiabatic system
Q ˙ − W ˙ s − F A 0 ∑ i = 1 N Θ i C P i [ T i − T i 0 ] − [ Δ H R x ∘ ( T R ) + Δ C P ( T − T R ) ] F A 0 X = 0
\dot Q
- \dot W_s
- F_{A0} \sum_{i=1}^{N} \Theta_i C_{P_i} \left[ T_{i} - T_{i0}\right]
- \left[ \Delta H_{Rx}^\circ(T_R) + \Delta C_{P}(T - T_R) \right] F_{A0} X
= 0
Q ˙ − W ˙ s − F A 0 i = 1 ∑ N Θ i C P i [ T i − T i 0 ] − [ Δ H R x ∘ ( T R ) + Δ C P ( T − T R ) ] F A 0 X = 0
Adiabatic system: Q ˙ \dot Q Q ˙ W ˙ s \dot W_s W ˙ s T i 0 = T 0 T_{i0} = T_0 T i 0 = T 0
− F A 0 ∑ Θ i C P i [ T i − T 0 ] − [ Δ H R x ∘ ( T R ) + Δ C P ( T − T R ) ] F A 0 X = 0
- F_{A0} \sum \Theta_i C_{P_i} \left[ T_{i} - T_{0}\right]
- \left[ \Delta H_{Rx}^\circ(T_R) + \Delta C_{P}(T - T_R) \right] F_{A0} X
= 0
− F A 0 ∑ Θ i C P i [ T i − T 0 ] − [ Δ H R x ∘ ( T R ) + Δ C P ( T − T R ) ] F A 0 X = 0
X E B = ∑ Θ i C P i [ T i − T 0 ] − Δ H R x ∘ ( T R ) + Δ C P ( T − T R )
X_{EB} = \frac { \sum \Theta_i C_{P_i} \left[ T_{i} - T_{0}\right] }
{- \Delta H_{Rx}^\circ (T_R) + \Delta C_{P}(T - T_R) }
X E B = − Δ H R x ∘ ( T R ) + Δ C P ( T − T R ) ∑ Θ i C P i [ T i − T 0 ]
Steady state energy balance
Constant heat capacity, adiabatic system
X E B = ∑ Θ i C P i [ T i − T 0 ] − Δ H R x ∘ ( T R ) + Δ C P ( T − T R )
X_{EB} = \frac { \sum \Theta_i C_{P_i} \left[ T_{i} - T_{0}\right] }
{- \Delta H_{Rx}^\circ (T_R) + \Delta C_{P}(T - T_R) }
X E B = − Δ H R x ∘ ( T R ) + Δ C P ( T − T R ) ∑ Θ i C P i [ T i − T 0 ]
In many instances, the Δ C P ( T − T R ) \Delta C_{P}(T - T_R) Δ C P ( T − T R ) Δ H R x ∘ ( T R ) \Delta H_{Rx}^\circ (T_R) Δ H R x ∘ ( T R )
T = T 0 + ( − Δ H R x ∘ ) X E B ∑ Θ i C P i
T = T_0 + \frac{ \left( -\Delta H^\circ_{Rx} \right) X_{EB}}{\sum \Theta_i C_{Pi}}
T = T 0 + ∑ Θ i C P i ( − Δ H R x ∘ ) X E B
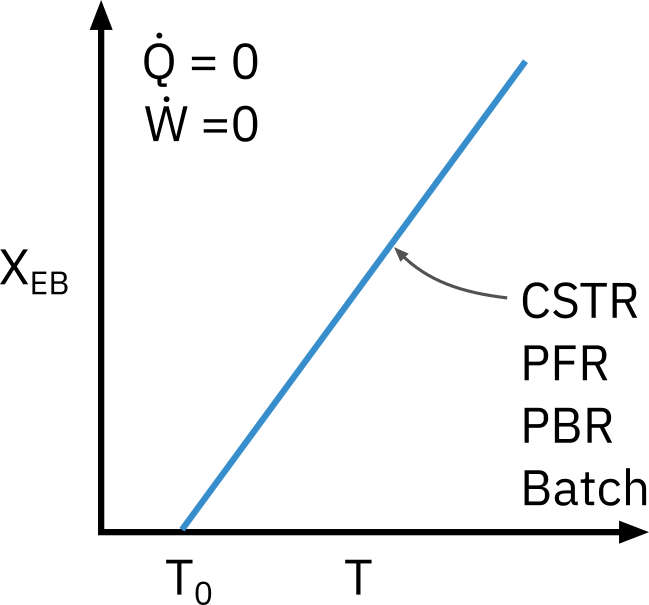
This equation applies to CSTR, PFR, PBR, and Batch reactors. It gives us the explicit relationship between X X X T T T
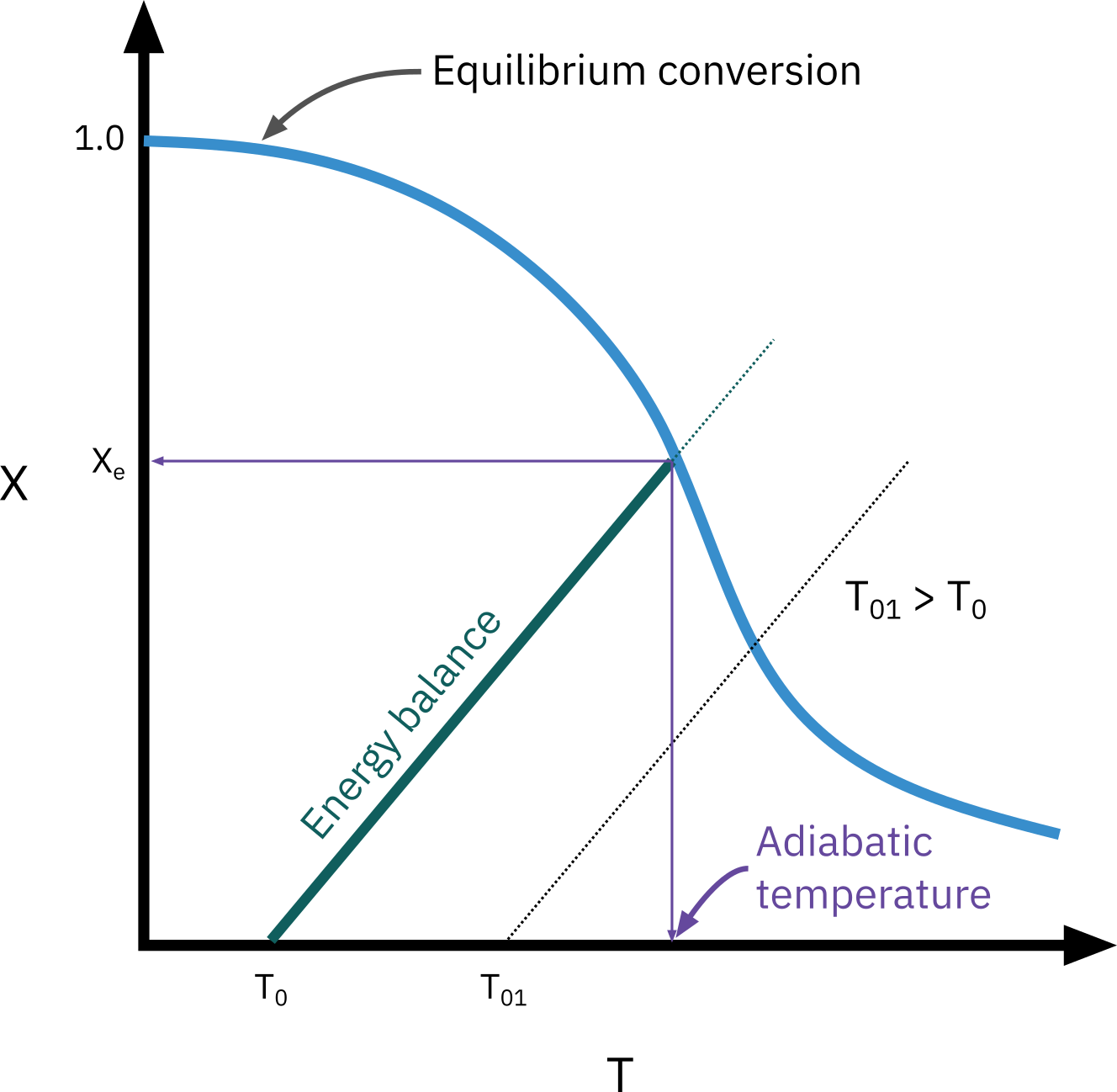
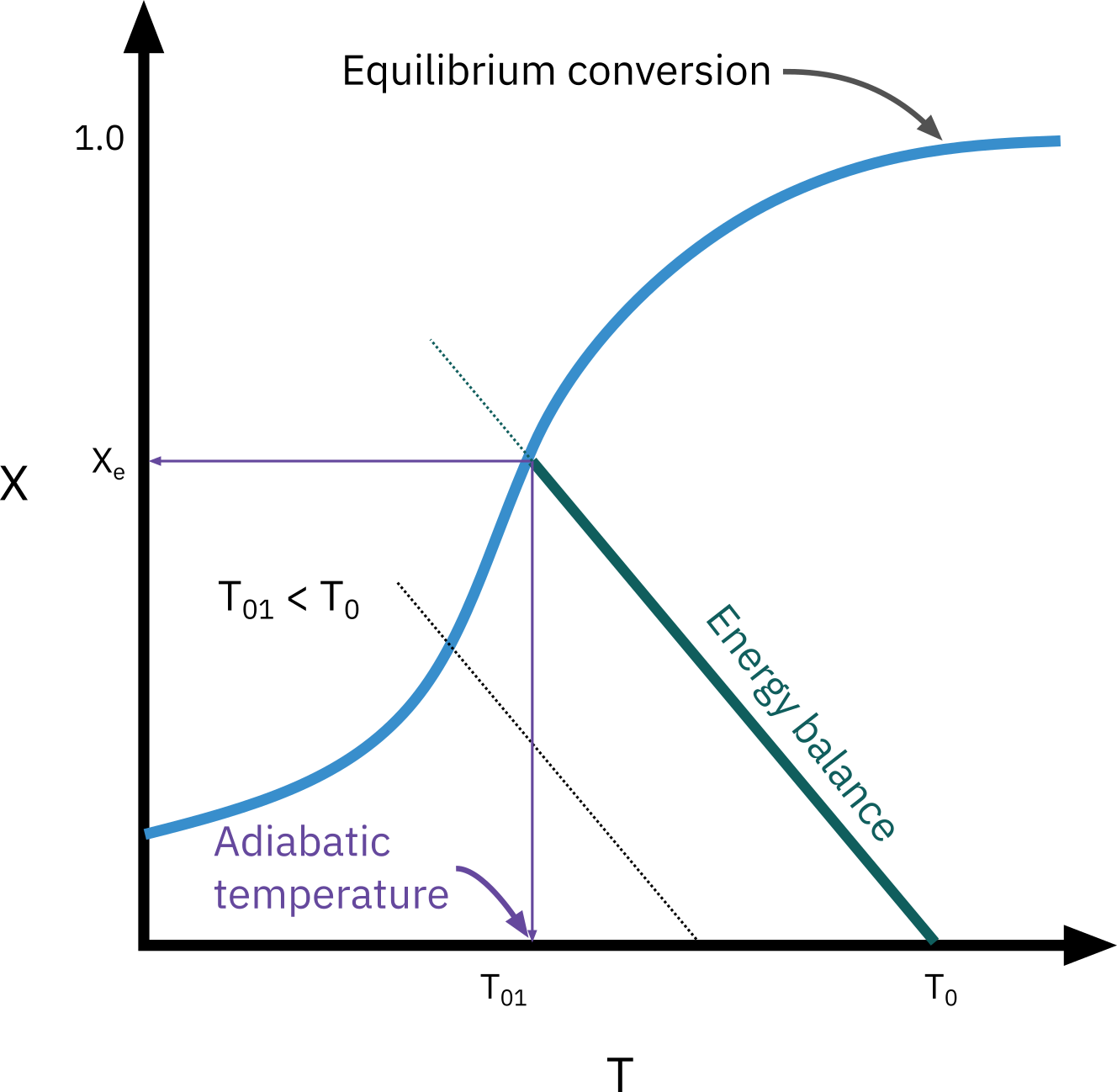
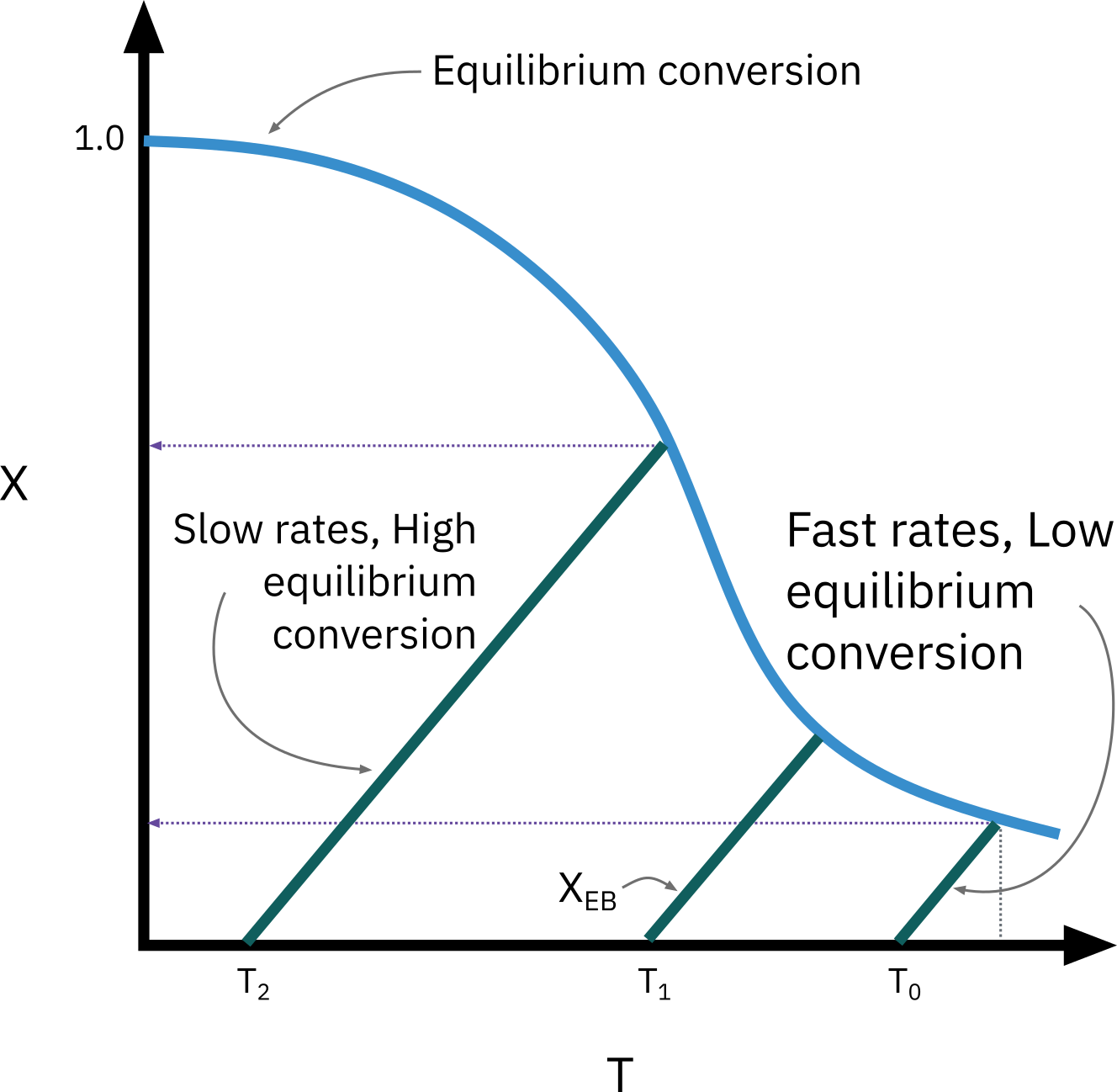
Adiabatic equilibrium conversion
Exothermic reactions
Endothermic reactions
The highest conversion that can be achieved in reversible reactions is the equilibrium conversion. For endothermic reactions, the equilibrium conversion increases with increasing temperature up to a maximum of 1.0. For exothermic reactions, the equilibrium conversion decreases with increasing temperature.
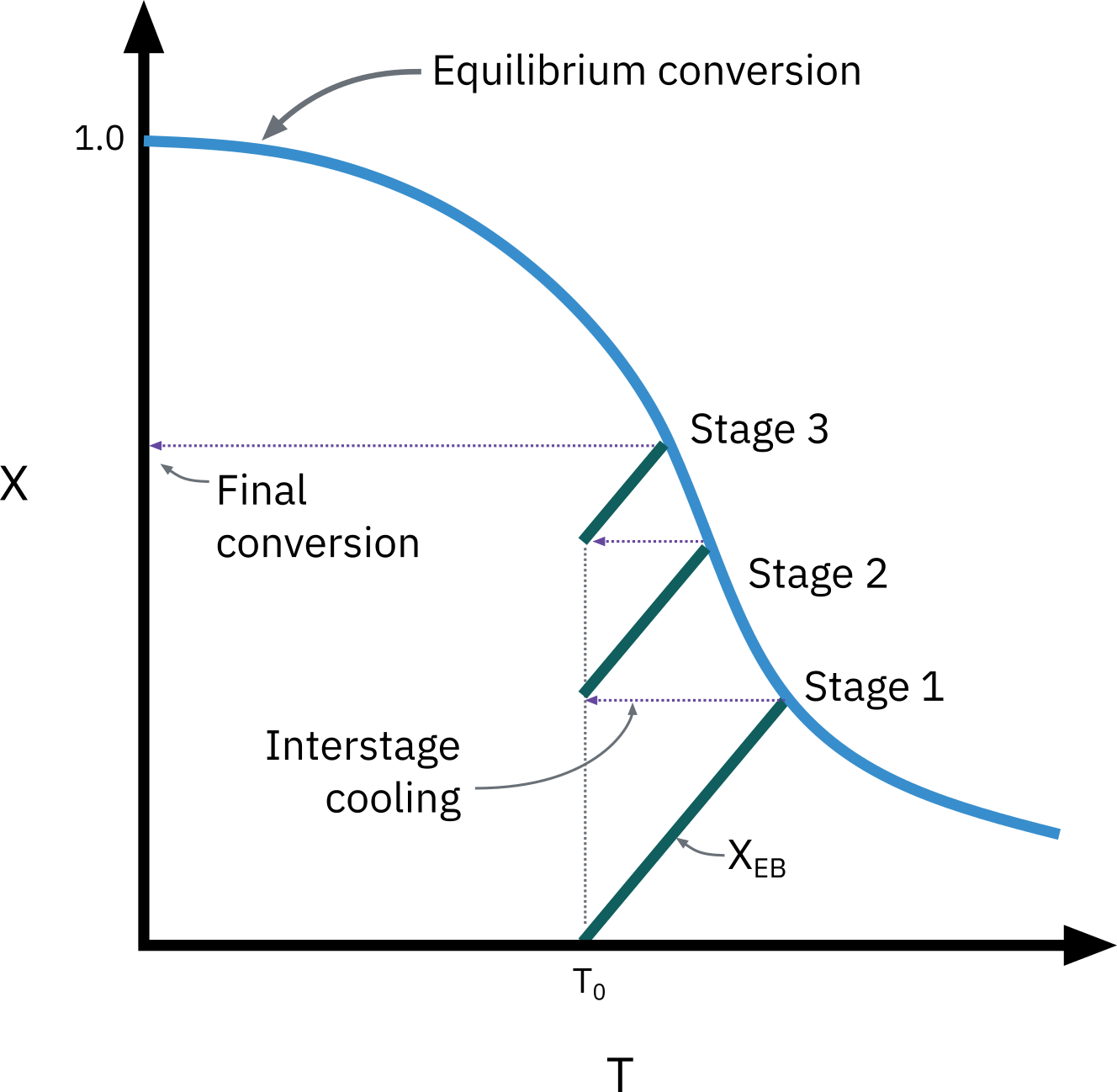
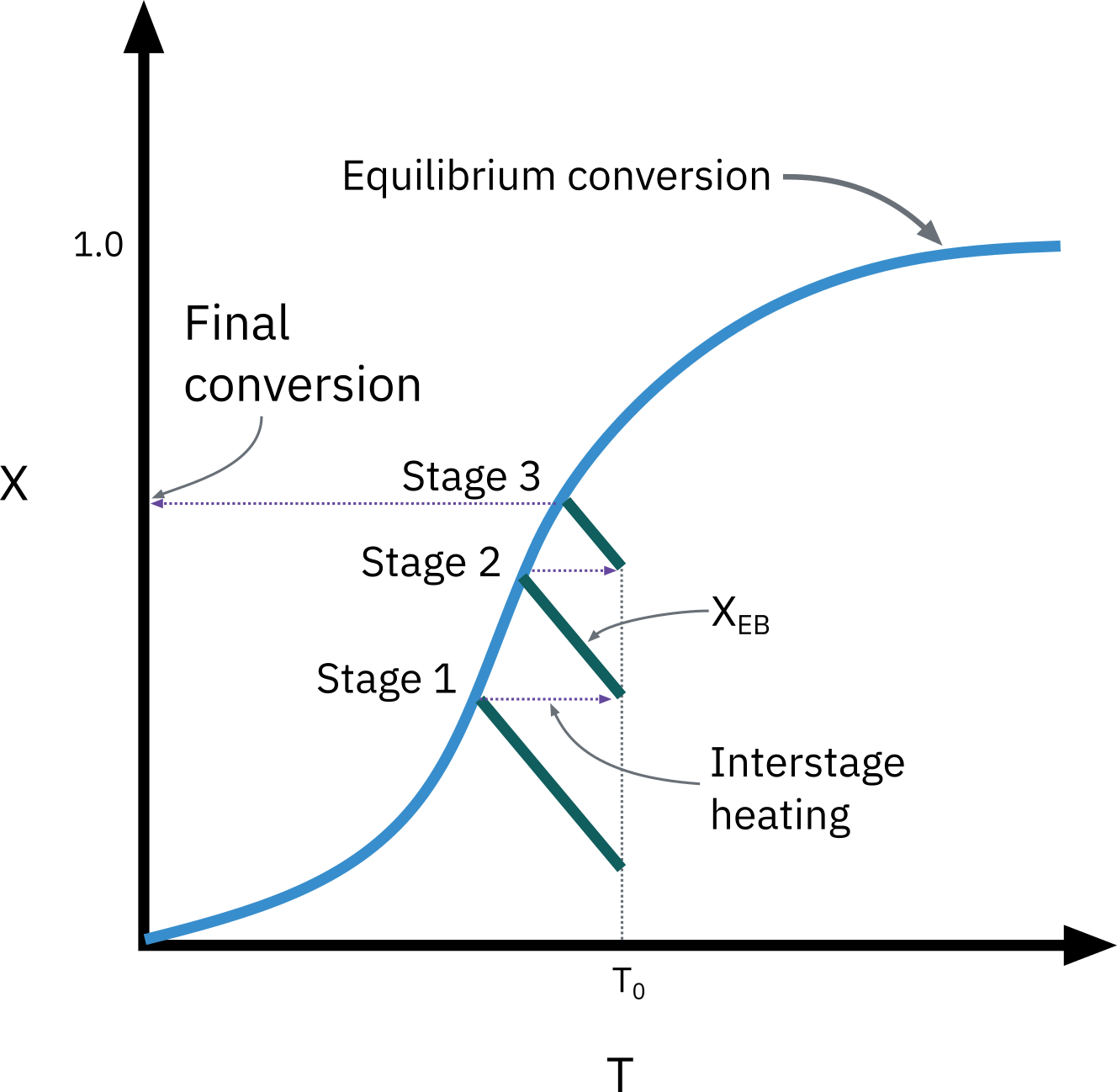
Reactor staging
Exothermic reactions
Endothermic reactions
Optimum feed temperature
Optimum feed temperature
Optimum feed temperature
Optimum feed temperature
Optimum feed temperature
Optimum feed temperature
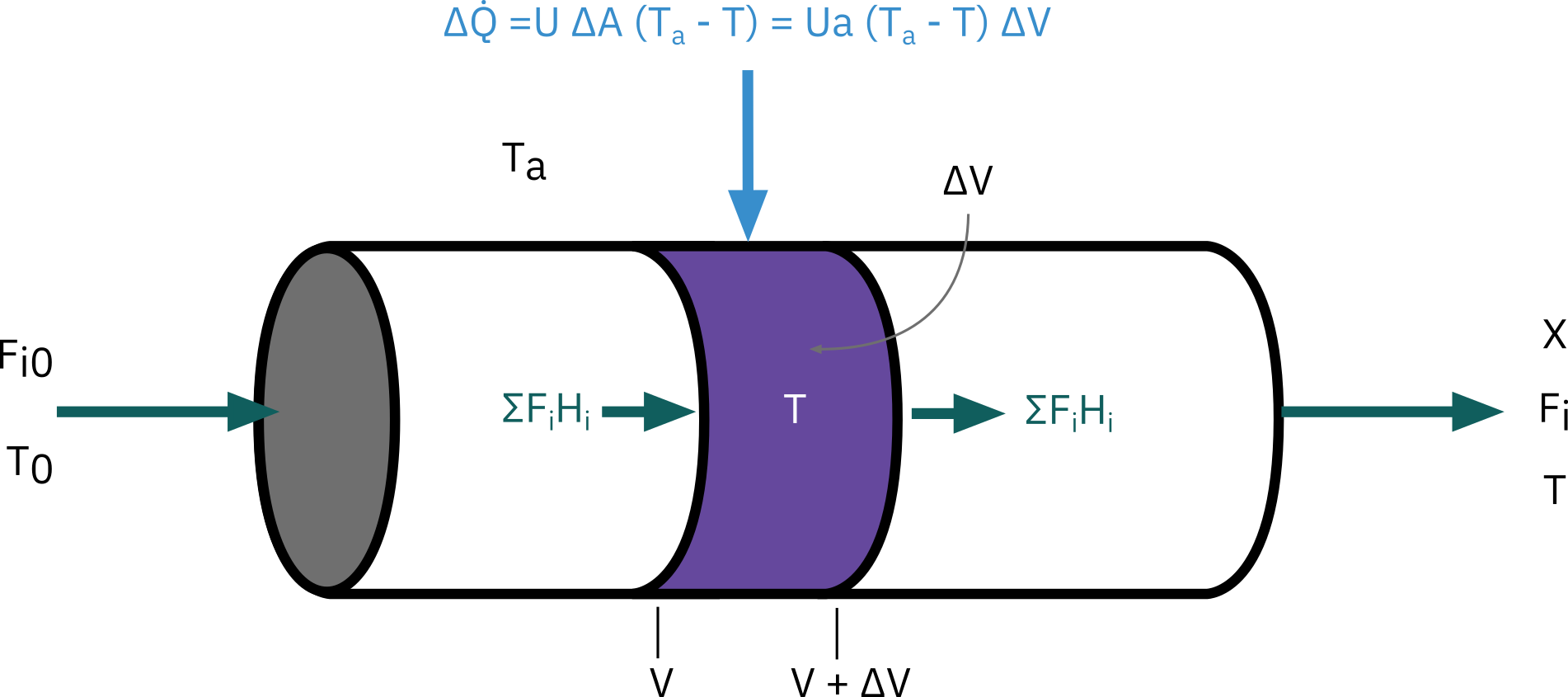
Steady-state tubular reactor with heat exchange
Energy balance over volume Δ V \Delta V Δ V
Δ Q ˙ + ∑ F i H i ∣ V − ∑ F i H i ∣ V + Δ V = 0
\Delta \dot Q + \sum F_i H_i |_V - \sum F_i H_i |_{V + \Delta V} = 0
Δ Q ˙ + ∑ F i H i ∣ V − ∑ F i H i ∣ V + Δ V = 0
Heat flow into the reactor Δ Q ˙ \Delta \dot Q Δ Q ˙
Δ Q ˙ = U Δ A ( T a − T ) = U a Δ V ( T a − T )
\Delta \dot Q = U \Delta A (T_a - T) = Ua \Delta V (T_a - T)
Δ Q ˙ = U Δ A ( T a − T ) = U a Δ V ( T a − T )
For tubular reactor a = 4 / D a = 4/D a = 4/ D
Dividing by Δ V \Delta V Δ V Δ V → 0 \Delta V \rightarrow 0 Δ V → 0
U a ( T a − T ) − d ∑ F i H i d V = 0
Ua (T_a - T) - \frac {d\sum F_i H_i}{dV} = 0
U a ( T a − T ) − d V d ∑ F i H i = 0
Steady-state tubular reactor with heat exchange
Expanding U a ( T a − T ) − ∑ d F i d V H i − ∑ F i d H i d V = 0
Ua (T_a - T) - \sum \frac {dF_i}{dV} H_i - \sum F_i \frac {dH_i}{dV} = 0
U a ( T a − T ) − ∑ d V d F i H i − ∑ F i d V d H i = 0
Mole balance: d F i d V = r i \frac{dF_i}{dV} = r_i d V d F i = r i d H i d V = C P i d T d V \frac{dH_i}{dV} = C_{P_i} \frac{dT}{dV} d V d H i = C P i d V d T
d T d V = r A Δ H R x ( T ) ⏞ Heat generated, Q g − U a ( T − T a ) ⏞ Heat removed, Q r Σ j n F j C P j
\frac{dT}{dV} = \frac{
\overbrace{r_A \Delta H_{Rx}(T)}^{\color{black}{\text{Heat generated, } Q_g }}
- \overbrace{Ua (T - T_a)}^{\color{black}{\text{Heat removed,} Q_r }}
}{ \Sigma_j^{n} F_j C_{P_j} }
d V d T = Σ j n F j C P j r A Δ H R x ( T ) Heat generated, Q g − U a ( T − T a ) Heat removed, Q r
Q g = r A Δ H R x ( T ) Q_g = r_A \Delta H_{Rx}(T) Q g = r A Δ H R x ( T )
positive for exothermic reactions; negative for endothermic reactions
When Q g > Q r Q_g > Q_r Q g > Q r
When Q g < Q r Q_g < Q_r Q g < Q r
For multiple reactions
Q g = ∑ i n r x n r i Δ H R x , i ( T ) Q_g = \sum_i^{n_{rxn}} r_i \Delta H_{Rx,i}(T) Q g = ∑ i n r x n r i Δ H R x , i ( T )
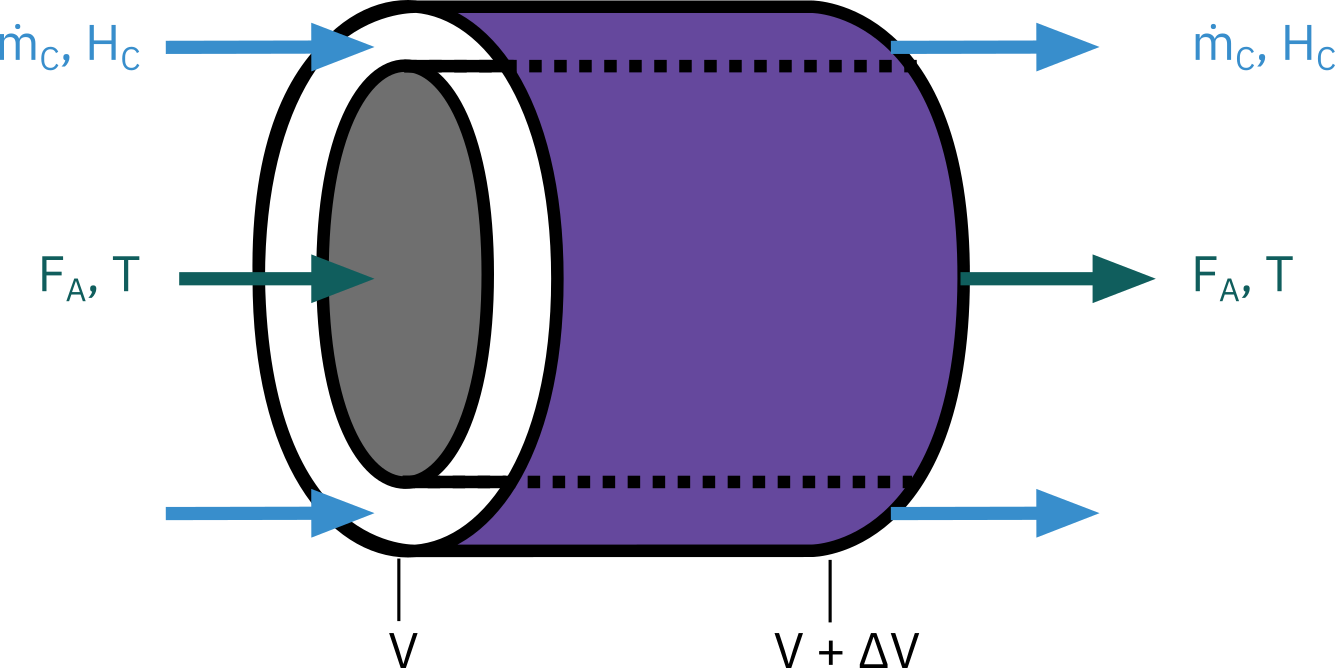
Balance on the heat-transfer fluid
The heat-transfer fluid will be a coolant for exothermic reactions and a heating medium for endothermic reactions.
If the flow rate of the heat-transfer fluid is sufficiently high with respect to the heat released (or absorbed) by the reacting mixture, then the heat-transfer fluid temperature will be virtually constant along the reactor. For all other cases, we need to write energy balance for the heat transfer fluid.
Rate of energy Rate of energy Rate of heat added in at V − out at V + Δ V + by conduction through = 0 the inner wall
\begin{aligned}
\text{Rate of energy}
& & \text{Rate of energy}
& & \text{Rate of heat added}
& &
\\
\text{in at } V
& \;\; - & \text{out at } V + \Delta V
& \;\; + & \text{by conduction through}
& \;\; = & 0
\\
& &
& & \text{the inner wall}
& &
\end{aligned}
Rate of energy in at V − Rate of energy out at V + Δ V + Rate of heat added by conduction through the inner wall = 0
m ˙ C H C ∣ V − m ˙ C H C ∣ V + Δ V + U a ( T − T a ) Δ V ⏟ Cocurrent flow = 0
\dot m_C H_C |_V
- \dot m_C H_C |_{ V + \Delta V }
+ \underbrace{U a (T - T_a) \Delta V}_{\color{RoyalBlue}{\text{Cocurrent flow}}}
= 0
m ˙ C H C ∣ V − m ˙ C H C ∣ V + Δ V + Cocurrent flow U a ( T − T a ) Δ V = 0
d T a d V = U a ( T − T a ) m ˙ C C P C
\frac{dT_a}{dV} =
\frac{U a (T - T_a)}
{ \dot m_C C_{P_C} }
d V d T a = m ˙ C C P C U a ( T − T a )
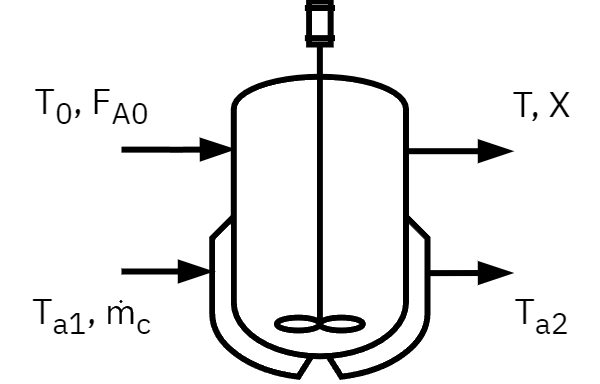
CSTR with heat effects
Steady state energy balance
Q ˙ − W ˙ s − F A 0 ∑ θ i C P , i ( T − T i 0 ) − [ Δ H R x ∘ ( T R x ) + Δ C P ( T − T R x ) ] F A 0 X = 0
\dot{Q} - \dot{W}_s - F_{A0}\sum \theta_i C_{P,i} (T - T_{i0}) -
[\Delta H^{\circ}_{Rx} (T_{Rx}) + \Delta C_{P} (T - T_{Rx})] F_{A0}X = 0
Q ˙ − W ˙ s − F A 0 ∑ θ i C P , i ( T − T i 0 ) − [ Δ H R x ∘ ( T R x ) + Δ C P ( T − T R x )] F A 0 X = 0
Shaft work, i.e., the work done by the stirrer or mixer in the CSTR on the reacting fluid inside the CSTR.
As W ˙ s \dot W_s W ˙ s
As F A 0 X = − r A V F_{A0} X = -r_A V F A 0 X = − r A V
Q ˙ − W ˙ s − F A 0 ∑ θ i C P , i ( T − T i 0 ) + ( r A V ) ( Δ H R x ) = 0
\dot{Q} - \dot{W}_s - F_{A0}\sum{\theta_i C_{P,i}(T - T_{i0})} +
(r_A V)(\Delta H_{Rx}) = 0
Q ˙ − W ˙ s − F A 0 ∑ θ i C P , i ( T − T i 0 ) + ( r A V ) ( Δ H R x ) = 0
A CSTR’s uniform temperature doesn’t ensure isothermal conditions; for that, the incoming feed must match the reactor’s internal temperature.
Except for processes with highly viscous fluids, the work done by the mixer is typically small enough to ignore.
The Q ˙ \dot Q Q ˙
Rate of energy in by flow:
m ˙ c C P , c ( T a 1 − T R ) \dot m_c C_{P,c} (T_{a1} - T_R) m ˙ c C P , c ( T a 1 − T R )
Rate of energy out by flow:
m ˙ c C P , c ( T a 2 − T R ) \dot m_c C_{P,c} (T_{a2} - T_R) m ˙ c C P , c ( T a 2 − T R )
Rate of heat transfer from exchanger to reactor:
U A ( T a 1 − T a 2 ) ln [ ( T − T a 1 ) ( T − T a 2 ) ]
\frac{UA(T_{a1} - T_{a2})}{\ln{\left[\frac{(T - T_{a1})}
{(T - T_{a2})}\right]}}
ln [ ( T − T a 2 ) ( T − T a 1 ) ] U A ( T a 1 − T a 2 )
An energy balance on the heat-exchanger fluid entering and leaving the exchanger
Rate of Rate of Rate of energy − energy − heat transfer = 0 in out from exchanger by flow by flow to reactor
\begin{aligned}
\text{Rate of}
& & \text{Rate of}
& & \text{Rate of}
& &
\\
\text{energy}
&\;\; - & \text{energy}
&\;\; - & \text{heat transfer}
&\;\; = & \text{0}
\\
\text{in}
& & \text{out}
& & \text{from exchanger}
& &
\\
\text{by flow}
& & \text{by flow}
& & \text{to reactor}
& &
\end{aligned}
Rate of energy in by flow − Rate of energy out by flow − Rate of heat transfer from exchanger to reactor = 0
Q ˙ = m ˙ c C P , c ( T a 1 − T a 2 ) = U A ( T a 1 − T a 2 ) ln ( T − T a 1 T − T a 2 )
\dot{Q} = \dot{m}_c C_{P,c} (T_{a1} - T_{a2}) =
\frac{UA (T_{a1} - T_{a2})}
{\ln\left(\frac{T - T_{a1}}{T - T_{a2}}\right)}
Q ˙ = m ˙ c C P , c ( T a 1 − T a 2 ) = ln ( T − T a 2 T − T a 1 ) U A ( T a 1 − T a 2 )
T a 2 = T − ( T − T a 1 ) exp ( − U A m ˙ c C P , c )
T_{a2} = T - (T - T_{a1}) \exp \left( \frac{-UA}{\dot{m}_c C_{P,c}} \right)
T a 2 = T − ( T − T a 1 ) exp ( m ˙ c C P , c − U A )
Q ˙ = m ˙ c C P , c ( T a 1 − T ) [ 1 − exp ( − U A m ˙ c C P , c ) ]
\dot{Q} = \dot{m}_c C_{P,c} \left( T_{a1} - T \right)
\left[ 1 - \exp \left( \frac{-UA}{\dot{m}_c C_{P,c}} \right) \right]
Q ˙ = m ˙ c C P , c ( T a 1 − T ) [ 1 − exp ( m ˙ c C P , c − U A ) ]
Conversion in a non-adiabatic CSTR
Ignoring W ˙ s \dot W_s W ˙ s
U A F A 0 ( T a − T ) − ∑ θ i C P , i ( T − T 0 ) − Δ H R x ∘ X = 0
\frac{UA}{F_{A0}} (T_a - T) - \sum \theta_i C_{P,i} (T - T_0) - \Delta H^{\circ}_{Rx} X = 0
F A 0 U A ( T a − T ) − ∑ θ i C P , i ( T − T 0 ) − Δ H R x ∘ X = 0
Solving for X X X
X = U A F A 0 ( T − T a ) + ∑ θ i C P , i ( T − T 0 ) − Δ H R x ∘ ( T R ) (1)
X = \frac{\frac{UA}{F_{A0}} (T - T_a) +
\sum \theta_i C_{P,i} (T - T_0)}
{-\Delta H^{\circ}_{Rx} (T_R)}
\tag{1} X = − Δ H R x ∘ ( T R ) F A 0 U A ( T − T a ) + ∑ θ i C P , i ( T − T 0 ) ( 1 )
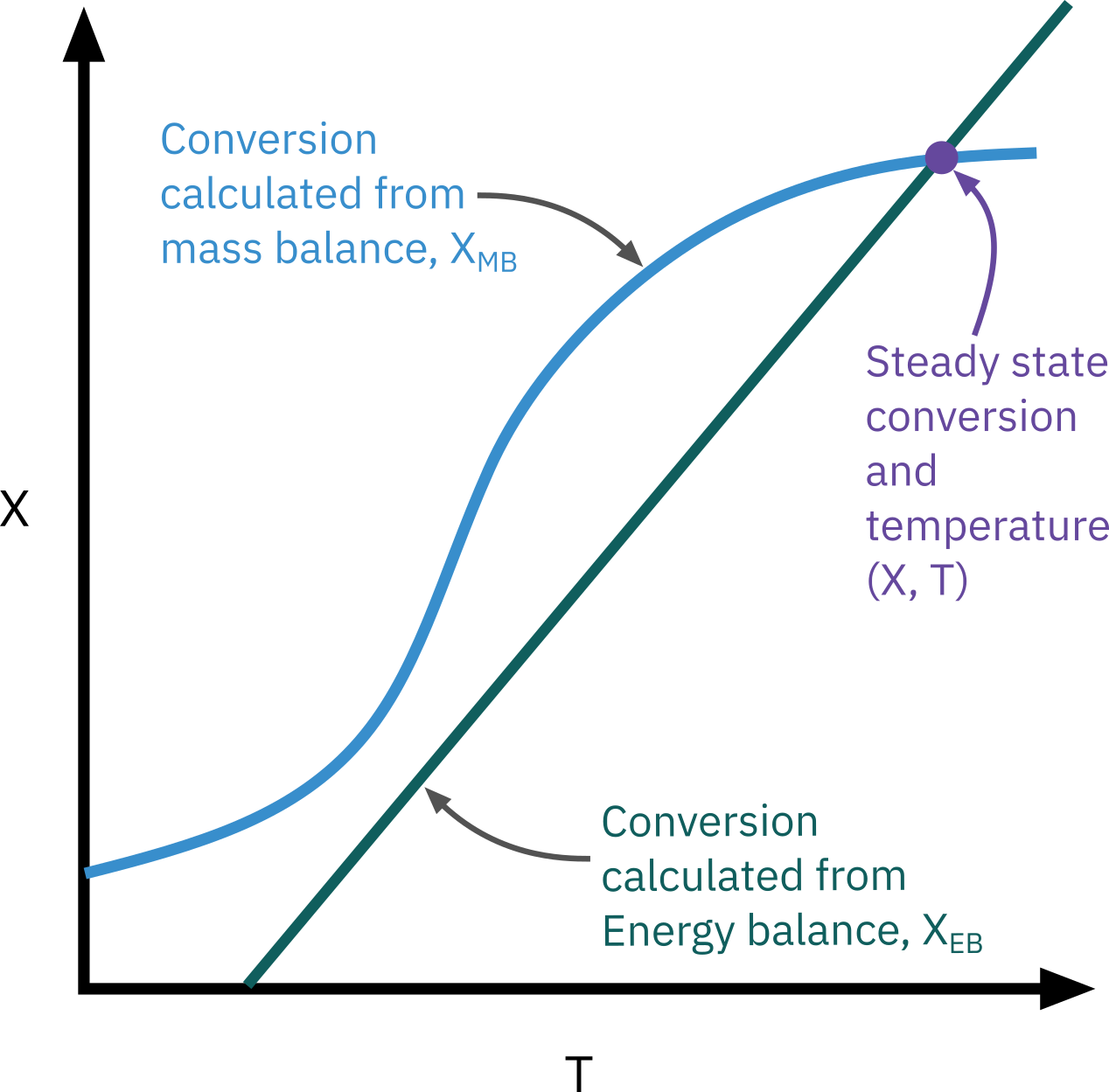
Couple Equation 1 with the mole balance equation V = F A 0 X − r A ( X , T ) V = \frac{F_{A0}X}{-r_A (X, T)} V = − r A ( X , T ) F A 0 X
Rearranging
X = C P , o ( 1 + κ ) ( T − T c ) − Δ H R x ∘ and T = T c + ( − Δ H R x ∘ ) ( X ) C P , o ( 1 + κ )
X = \frac{C_{P,o} (1 + \kappa) (T - T_c)}{-\Delta H^{\circ}_{Rx}}
\quad \text{and} \quad
T = T_c + \frac{(-\Delta H^{\circ}_{Rx})(X)}{C_{P,o}(1 + \kappa)}
X = − Δ H R x ∘ C P , o ( 1 + κ ) ( T − T c ) and T = T c + C P , o ( 1 + κ ) ( − Δ H R x ∘ ) ( X )
Where C P , o = ∑ θ i C P , i , κ = U A F A 0 C P , o ,and T c = κ T a + T 0 1 + κ
\text{Where} \quad
C_{P,o} = \sum \theta_i C_{P,i}
\quad \text{,} \quad
\kappa = \frac{UA}{F_{A0} C_{P,o}}
\quad \text{,and} \quad
T_c = \frac{\kappa T_a + T_0}{1 + \kappa}
Where C P , o = ∑ θ i C P , i , κ = F A 0 C P , o U A ,and T c = 1 + κ κ T a + T 0
Conversion in a non-adiabatic CSTR