Multiple reactions
Chemical Reaction Engineering
Introduction
- Usually, more than one reaction occurs within a chemical reactor
- Minimization of undesired side reactions that occur with the desired reaction contributes to the economic success of a chemical plant
- Goal: determine the reactor conditions and configuration that maximizes product formation
- Reactor design for multiple reactions
- Parallel reactions
- Series reactions
- Independent reactions
- More complex reactions
- Use of selectivity factor to select the proper reactor that minimizes unwanted side reactions
With multiple reactions, either molar flow or number of moles must be used in setting up the balance equations (no conversion!)
Isothermal reactions in PBR: molar flow rates
Second order reaction aA+bBcC+dD
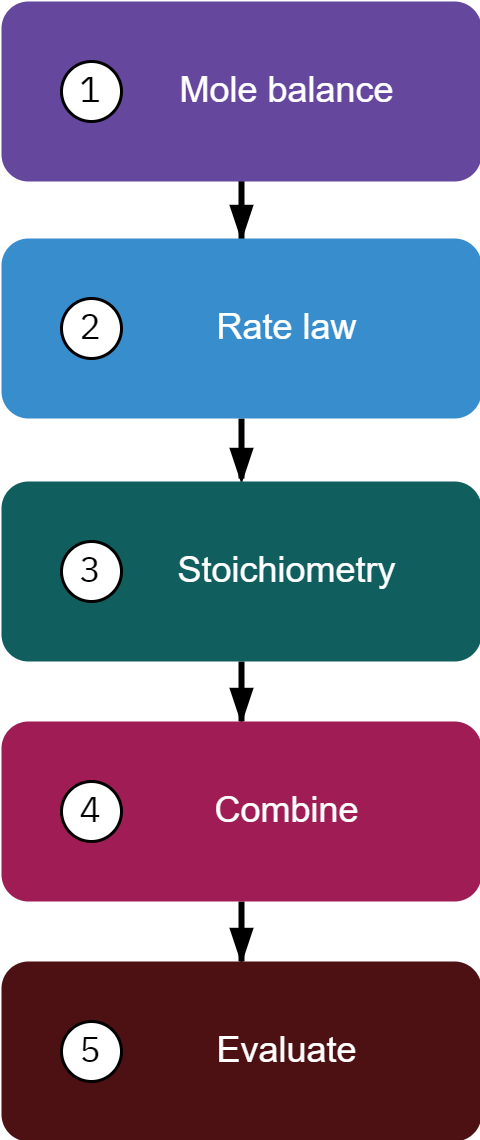
Mole balance: Write balance for each species i=1 to N
dWdFi=ri′
Rate law
−rA′=kCAαCBβ;−arA′=−brB′=crC′=drD′
Stoichiometry Ci=(1+ϵX)CA0(Θi+νiX)(P0P)(TT0)
Pressure: dWdP=−2pα(T0T)FT0FT
Total molar flow rate: FT=∑i=1NFi
Combine:
Collate all equations from steps 1 to 3 to yield a system of equations
Evaluate:
Use ODE solver to solve the system of equations obtained in step 4.
Parallel reactions
Competing reactions
Reactant is consumed by two different pathways to form different products
AkX1DAkX2U
Examples
Ethylene oxidation
CX2HX4+OX2CX2HX4+OX2CX2HX4O2COX2+2HX2Oethylene oxidecomplete combustion
Fischer Tropsch synthesis
CO+3HX2CO+2HX2kX1CHX4+HX2OkX2[CXnHX2n]Xn+HX2O
Series reactions
Consecutive reactions
Reactions where reactant forms an intermediate product, which reacts further to form another product.
AkX1DkX2U
Example
- Ethylene oxide (EO) + Ammonia
CX2HX4O+NHX3(HOCHX2CHX2)NHX2+CX2HX4O(HOCHX2CHX2)X2NH+CX2HX4O(HOCHX2CHX2)NHX2desired product(HOCHX2CHX2)X2NH(HOCHX2CHX2)X3N(mono ethanolamine)(di ethanolamine)(tri ethanolamine)
Independent reactions
Reactions that occur at the same time
Neither the products nor the reactants react with themselves or one another
AkX1DCkX2U
Example: Cracking of crude oil
Hundreds of reactions
CX15HX32CX8HX18CX12HX26+CX3HX6CX6HX14+CX2HX2
Complex reactions
Multiple reactions involving a combination of series, parallel, and/or independent reactions
A+BA+CEkX1C+DkX2EkX3G
Example: Formation of butadiene from ethanol
CX2HX5OHCX2HX5OHCX2HX4+CHX3CHOCX2HX4+HX2OCHX3CHO+HX2CX4HX6+HX2O
Desired and undesired reactions
Parallel reactions AADu←desired product←undesired byproduct
Series reactions Adesired productBU
Minimize formation of U and maximize formation of D

Greater the amount of U ← lower production of desired product, higher cost of separation ← lower profits
Instantaneous selectivity (S)
- Instantaneous selectivity of D with respect to U is the ratio of the rate of formation of D to the rate of formation of U
SD/U=rUrD=rate of formation of Urate of formation of D
- Gives insights in choosing reactors, operating conditions, and reaction schemes that will maximize profit.
- Used to guide initial selection of reactor system.
- Final selection is made after calculating the overall selelctivity for the reactor and operating conditions chosen.
Overall selectivity (Sˉ)
SˉD/U=FUFD=Exit molar flow rate of UExit molar flow rate of D
For CSTR:
FD=rDV
FU=rUV
∴FD/FU=rD/rU=SD/U=SˉD/U
For batch reactor:
SˉD/U=ND/NU
ND,NU: Number of moles of D and U at the end of the reaction.
Yield (Y)
Instantaneous yield (YD)
Ratio of the reaction rate of a given product to the reaction rate of key reactant A
YD=−rArD=Rate of consumption of ARate of formation of D
Overall yield (YˉD)
Ratio of moles of product formed at the end of the reaction to the number of moles of the key reactant A, that have been consumed.
YˉD=For CSTRFA0−FAFD=For batch reactorNA0−NAND
- Overall selectivities (Sˉ) and yields (Yˉ) are important in determining profits
Conversion (X)
Gives insight into problem
Often conflicts with selelctivity
Ideal world ⇒ make as much D as possible simultaneously minimize U
Practical experiece ⇒ Greater the conversion, more the undesired product
Not used in solving multiple reaction problems, but calculated later for analysis
For species A XA=Flow systemFA0FA0−FAXA=Batch systemNA0NA0−NA
For species B XB=Flow systemFB0FB0−FBXB=Batch systemNB0NB0−NB
Derive expression for conversion for a semibatch system where B is fed to A
Algorithm for multiple reactions

Number each and every reaction separately.
Mole balance on each and every species.
Fj0−Fj+∫VrjdV=dtdNj
For every reaction write rate law. Calculate the net rate of reaction and relative rates
For component j: rj=∑i=1Nri,j
Stoichiometry
Combine:
Collate all equations from steps 2 to 4 to yield a system of equations
Evaluate:
Use ODE solver to solve the system of equations obtained in step 4.
Can be applied to parallel, series, independent, and complex reactions.
For liquid systems concentration is usually preferred variable for mole balance.
Reactor configurations





Parallel reactions: Selectivity
Consider two competing reactions
ADAU(desired)(undesired)rD=kDCAα1rU=kUCAα2
Net rate of disappearance of A
−rA=rD+rU=kDCAα1+kUCAα2
SD/U=rUrD=kUkDCAα1−α2
α1, α2 are positive orders
Case 1: α1>α2
α1−α2=a
SD/U=kUkDCAa
To maximize selectivity we wan to carry out the reaction in a manner that will keep CA as high as possible during the reaction.
Use PFR or batch reactor
Gas phase: Use high pressure, run without inerts
Liquid phase: minimize diluent


Case 2: α1<α2
α2−α1=b
SD/U=kUkDCAb1
To maximize selectivity we wan to carry out the reaction in a manner that will keep CA as low as possible during the reaction.
Use CSTR or dilute feed stream
Recycle reactor
- Product stream can act as a diluent


Effect of temperature
Need information on activation energy
Sensitivity to temperature for fixed concentrations (CAa is constant)
SD/U≈kUkD=AUADe−[(ED−EU)/RT]
ED>EU
- kD increases more rapidly than kU with increase in temperature
- Perform the reaction at highest possible temperature
ED<EU
kU increases more rapidly than kD with increase in temperature
Reaction should be carried out at lower temperature to maximize SD/U
The temperature should not be very low as it might affect reaction extent. Reaction may not proceed at low temperature.
Two simultaneous reactions and two reactants
Consider two competing parallel reactions
A+BDA+BU(desired)(undesired)rD=kDCAα1CBβ1rU=kUCAα2CBβ2
Net rate of disappearance of A
−rA=rD+rU=kDCAα1CBβ1+kUCAα2CBβ2
Selectivity
SD/U=rUrD=kUkDCAα1−α2CBβ1−β2
- Selectivity depends on the orders α1, α2, β1, β2
- Several reactor combinations exist
Two simultaneous reactions and two reactants
α1−α2=a;β1−β2=b
α1>α2; β1>β2
SD/U∝CAaCBb
Use high CA,CB
Configurations:
- Tubular reactor
- Batch reactor
- High pressure (gas phase)
- Reduce inerts
α1>α2; β1<β2
SD/U∝CBbCAa
Use high CA, low CB
Configurations:
- Semi batch reactor with B fed slowly into large amt of A
- Membrane / tubular reactor with side stream of B continually fed into the reactor
- Series of small CSTRs (A is fed only to the first reactor, B is fed to each reactor)
Two simultaneous reactions and two reactants
α1−α2=a;β1−β2=b
α1<α2; β1>β2
SD/U∝CBbCAa
Use high CB, low CA
Configurations:
- Semi batch reactor with A fed slowly into large amt of B
- Membrane / tubular reactor with side stream of A continually fed into the reactor
- Series of small CSTRs (B is fed only to the first reactor, A is fed to each reactor)
α1<α2; β1<β2
SD/U∝CAaCBb1
Use low CA,CB
Configurations:
CSTR
Feed diluted streams with inerts
Low pressure (gas phase)
Tubular reactor with large recycle ratio
Can be used for highly exothermic reactions. The recycle stream is cooled and returned to the reactor to dilute and cool inlet stream.
Such configuration helps in avoiding hotspots and runaway reactions.
Reactions in series
The most important variable is time
- Batch time (real time)
- Space time (continuous reactor)
Consider: AkX1BkX2C.
B is the desired product
If k1≪k2: First reaction is slow
- Extremely difficult to produce significant amount of B.
If k1≫k2: First reaction is fast
- Large yield of B can be achieved.

If reaction is allowed to proceed for a long time, desired product B will be converted to undesired product C.
Accuracy of prediction for time required to carry out the reaction is vital.
Reactions in series
Consider the reaction
AkX1BkX2C.
B is the desired product, C is waste product.
We are interested in
- Concentration vs. time profile
- Maximum concentration of B
- Quench time (time to stop when CB is maximum)
- Overall selectivity and yield.
Number of reactions
The series reaction can be written as two reactions
AB;−r1A=k1CA.
BC;−r2B=k2CB.
Mole balance dtdNi=riV
For constant volume batch reactor
dtdCA=rA=−k1CA(1)
dtdCB=rB=k1CA−k2CB(2)
dtdCC=rC=k2CC(3)
- Solve Equation 1, Equation 2, and Equation 3 simultaneously to obtain concentration profile.


Chemical Reaction Engineering