Rate law and stoichiometry
Chemical Reaction Engineering
Rate law
Reactor mole balance in terms of X
| Reactor | Mole balance |
|---|---|
| Batch | NA0dtdX=rAV |
| CSTR | V=−rAFA0X |
| PFR | FA0dVdX=−rA |
| PBR | FA0dWdX=−rA′ |
Rate law
The algebraic expression for the reaction rate equation, rj.
−rA=ftemperaturedependentterms,concentrationdependentterms=f(T,C)]
How to derive an equation for –rA in terms of XA
Relate all rj to Cj ⇒ Rate law
Relate all Cj to V or υ ⇒ Stoichiometry
Relate V or υ to XA ⇒ Stoichiometry
Put together
rA=f(T,XA)
Basic definitions
Homogeneous reaction: involves only one phase.
Heterogeneous reaction: involves more than one phase. Reaction usually occurs at the interface.
Irreversible reaction: proceeds in only one direction. Continues until one of the reactants is exhausted.
Reversible reaction: proceeds in either direction. Continues until equilibrium concentration is reached.
No reaction is completely irreversible. For irreversible reactions equilibrium point lies far to the product side.
Reaction rate
Rate laws: are the algebraic equations that apply to a given reaction
Relative rates: how fast one species appears or disappears relative to the other species in a given reaction.
Net rate of formation of a given species (e.g., A): is the sum of the rate of the reactions of A for all the reactions in which A is either a reactant or product.
Molecularity of reaction
Number of atoms, ions, or molecules involved (colliding) in a reaction step
Unimolecular: One molecule colliding in one reaction step
radioactive decay
alpha decay of Uranium-238 emitting an alpha particle (a helium nucleus) and formation of Thorium-234.
X92X2922UX238X90X2902ThX234+X2X222HeX4
rate of disappearance of U ⇒−rU=kCU
Bimolecular: Two molecule colliding in one reaction step
Only true bimolecular reactions are those involving collision with free radicals
initiation step of the free radical bromination of ethane
Br⋅+CX2HX6HBr+CX2HX5⋅
−rBr⋅=kCBr⋅CCX2HX6
Trimolecular: Three molecule colliding in one reaction step
The probability of trimolecular reaction is almost non-existent
In most instances the reaction pathway follows a series of bimolecular reactions.
Relative rates of reaction
How fast one species appears or disappears relative to the other species in a given reaction.
Can be obtained from the ratio of stoichiometric coefficients
Consider a reaction
aA+bBcC+dD
For every mole of A consumed ac moles of C appear
rC=[rate offormation of C]=ac[rate ofdisappearance of A]=ac(−rA)
rC=a−crA Similarly, rC=dcrD
Aa−rA+=ab Bb−rB=ac CcrC+=ad DdrD
In class exercise: relative rates
- For the reaction 2A+B3C, calculate rates of reaction of B and C if −rA=10mol/(dm3s)
In class exercise: relative rates
- NOX2 is being formed at a rate of 4 mol/(m3s) in the reaction 2NO+OX22NOX2. Calculate the rate of consumption of NO, and OX2.
Rate law
The rate of reaction depends on collision frequency of the molecules.
Concentration and temperature
Molecular collision frequency ∝ concentration
⇒ Rate of reaction ∝ concentration. −rA=f(CA,CB,...)for const. T
As temperature increases, collision frequency increases
⇒ Rate of reaction ∝ temperature. −rA=f([T],[CA,CB,...])
For many reactions
−rA=kA(T)f(CA,CB,...)
Specific rate of reaction (rate constant), kA(T)
Proportionality constant in the rate equation
Depends on the temperature (following the Arrhenius equation), the presence of a catalyst, and other environmental conditions but is independent of the reactant concentrations.
Power law model
Dependence of −rA on concentration of species present (f(Cj)) is almost always determined by experimental observations.
Functional dependence on concentration may be postulated by theory
⇒ Experiments are required to confirm the proposed form
Power law model is most common general forms of the rate law
−rA=kACAαCBβ
Order of reaction: the powers to which the concentrations are raised
- The reaction is α order with respect to A and β order with respect to B.
- Overall order of reaction n=α+β.
kA: Specific reaction rate
Units of kA=time(conc)1−n
Order of reaction
AProducts
| Reaction order | Rate equation | Units of kA |
|---|---|---|
| 0th order | −rA=kA | {k}=volume smol |
| 1st order | −rA=kACA | {k}=s1 |
| 2nd order | −rA=kACA2 | {k}=mol svolume |
| 3rd order | −rA=kACA3 | {k}=s(volume/mol)2 |
Elementary reaction
A reaction involving single step is called an elementary reaction.
Stoichiometric coefficients in the reaction are equal to the powers in the rate law.

2NO+OX22NOX2
This reaction is not elementary, but under some conditions it follows an elementary rate law
−rNO=kNOCNO2CO2
⇒ Elementary as written
CO+ClX2COClX2
This reaction is non-elementary
−rCO=kCOCCOCCl23/2
1st order with respect to CO and 3/2 order with resect to ClX2 (5/2 order overall)
⇒ Non-elementary reaction
Complex rate expression
2NX2O2NX2+OX2−rN2O=1+k′CO2kN2OCN2O
Rate expression cannot be separated into solely temperature dependent and concentration dependent terms.
Overall reaction order cannot be stated
Only undrer limiting circumstances we can speak of reaction order
for 1>>k′CO2;−rN2O=kN2OCN2O ⇒ ‘Apparent’ first order reaction
for 1<<k′CO2;−rN2O=kN2OCO2CN2O ⇒ ‘Apparent’ 0th order reaction
Reaction is −1th order with OX2 and 1st order with NX2O.
This kind of rate expression is common for liquid and gaseous reactions promoted by solid catalysts.
Heterogeneous reactions
Historically for many gas-solid catalyzed reactions it is customary to write rate laws in terms of partial pressures rather than concentrations.
Weight of catalyst is important rather than reactor volume (⇒−rA′)
Hydromethylation of toluene
CX6HX5CHX3+HX2catCX6HX6+CHX4
−rT′=1+KBPB+KTPTkPH2PT
- ’ (prime): indicates typical units are in /g-cat
- P: partial pressures (kPa or atm)
- K: adsorption constant (1/kPa or atm−1)
- [K]=kg cat⋅s⋅kPa2mol toluene
Use ideal gas law to express the reaction in terms of concentrations: Pi=CiRT
Reversible reaction
All rate laws must reduce to thermodynamic relationships relating the reacting species concentrations at equilibrium
At equilibrium rate of reaction is zero for all the species (−rA≡0)
For a reaction aA+bBcC+dD
Kc=CAeaCBebCCecCDed[Kc]=(volumemol)c+d−a−b
For elementary reaction AkfkrB
−rA=k(CA−KcCB);Kc=krkf
In class exercise: rate law
Determine the rate law for the reaction described in each of the cases below involving species A, B, and C. The rate laws should be elementary as written for reactions that are either of the form AB or A+BC.
- The units of the specific reaction rate are k=[mol hdm3].
- The units of the specific reaction rate are k=[kg−cat h (atm)2mol].
- The units of the specific reaction rate are k=[h1].
- The units of a nonelementary reaction rate are k=[dm3 hmol].
In class exercise: rate law
For the reaction CX6HX6kBk-BCX6HX4+HX2(BD+HX2)
determine the rate expression for disappearance of benzene (−rB). Assume both the forward and reverse reactions are elementary.
Equilibrium constant
van’t Hoff equation
When there is no change in number of moles and heat capacity term ΔCp=0
Kc(T)=Kc(T1)exp[RΔHRx∘(T11−T1)]
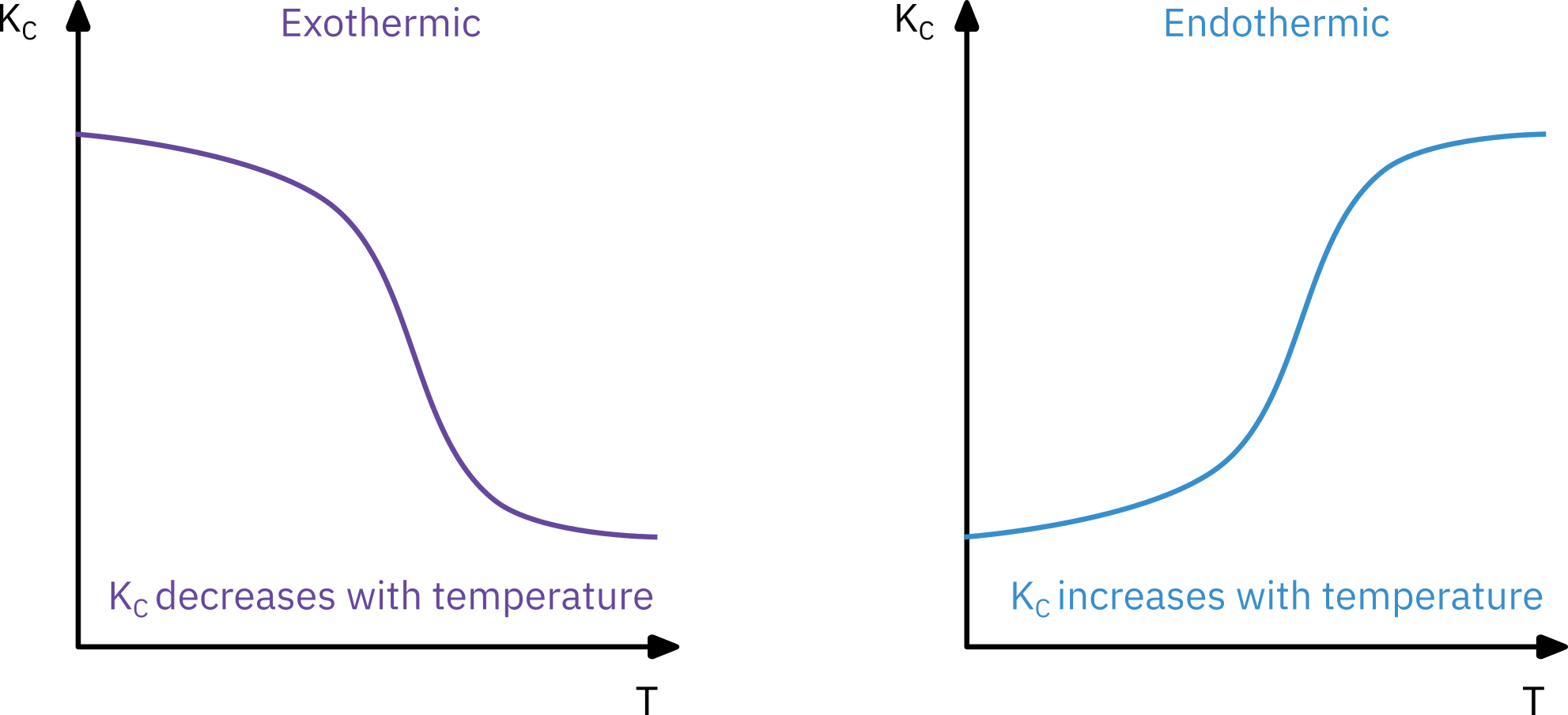
The reaction rate constant (kA)
⇒ Not a constant. Just independent of concentration
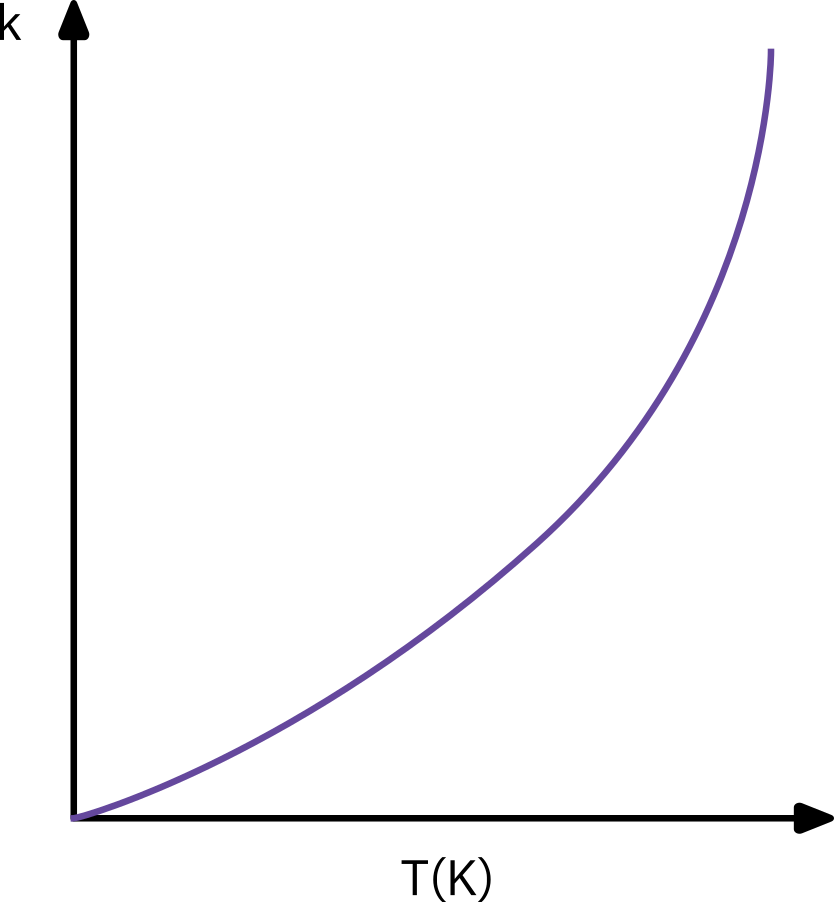
Arrhenius equation
kA(T)=AeRT−E⇒k0e−E/RT
- k0,A: Preexponential factor/ frequency factor
- E: Activation energy (J/mol) or (cal/mol)
- R: Gas constant (= 8.314 J/mol K)
- T: Absolute temperature (K)
Empirically varified over a large temperature range
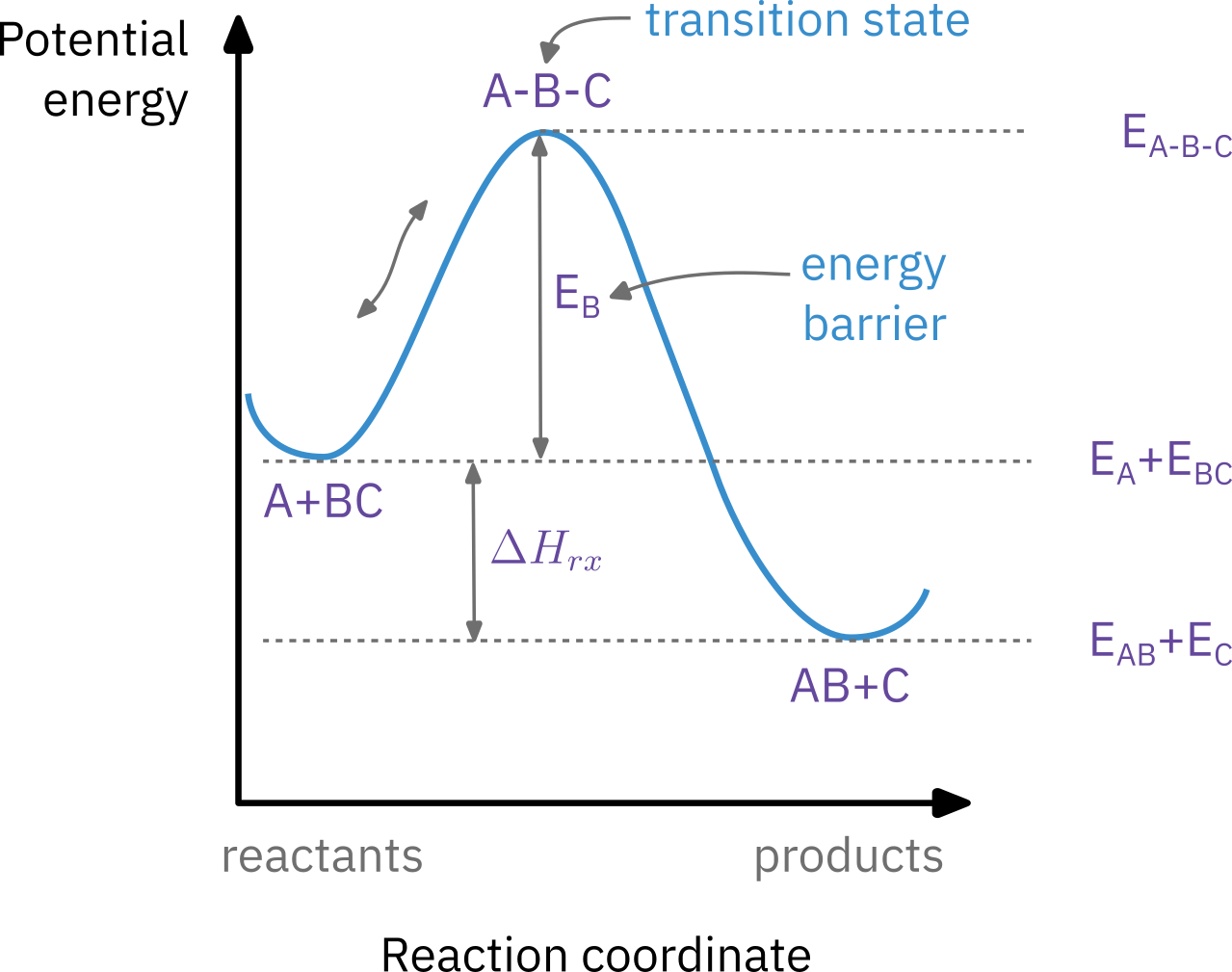
Activation energy
Barrier to energy transfer (from kinetic energy to potential energy) between reacting molecules that must must be overcome.
Minimum increase in potential energy of the reactants that must be provided to transform the reactants into products
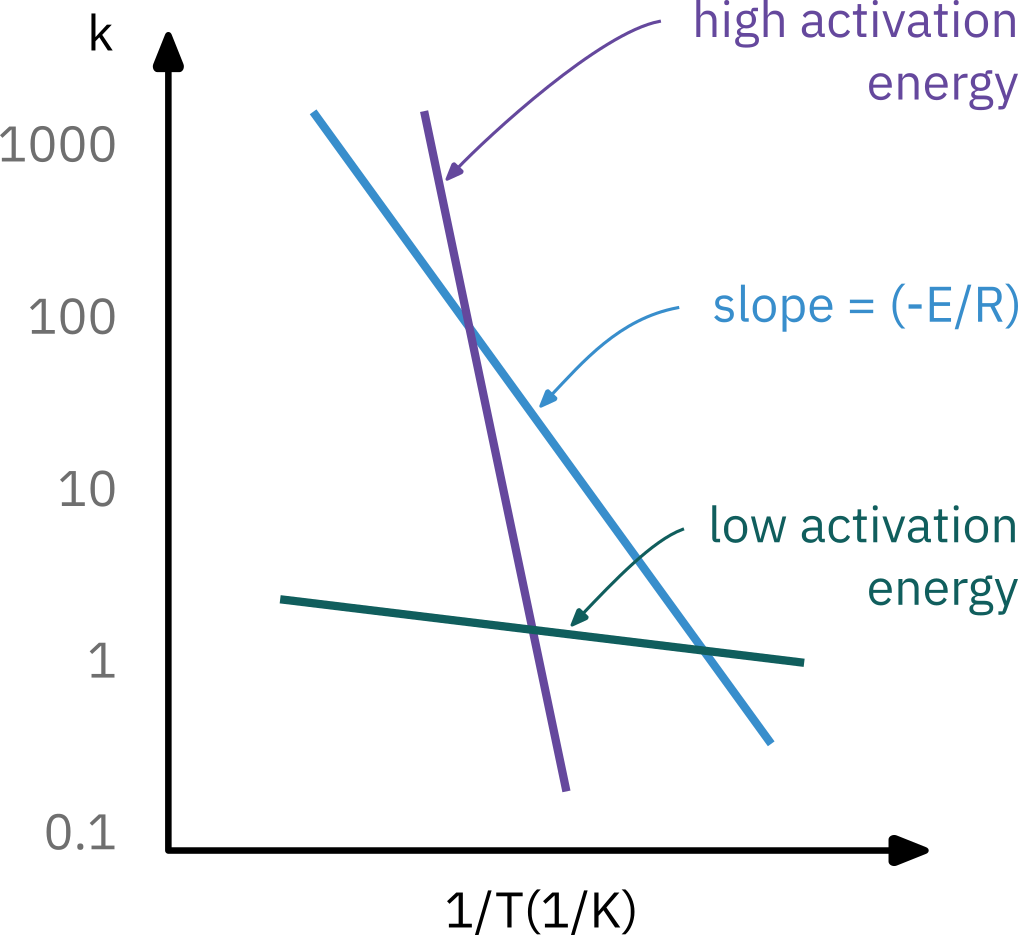
Determining activation energy
Experimentally measuring reaction rate at different temperatures
kA(T)=Ae−E/RT
Taking natural log
lnkA=lnA−RE(T1)
k(T)=k(T0)eRE(T01−T1)
- Rule of thumb: Reaction rate doubles every 10 °C rise in temperature
Arrhenius plot
In class exercise: activation energy
The decomposition of benzene diazonium chloride to give chlorobenzene and nitrogen
CX6HX5NX2ClCX6HX5Cl+NX2
follows first order kinetics. The rate constant data at different temperatures is given in Table 1. Calculate the activation energy.
| k(s−1) | 0.00043 | 0.00103 | 0.00180 | 0.00355 | 0.00717 |
| T(K) | 313.0 | 319.0 | 323.0 | 328.0 | 333.0 |
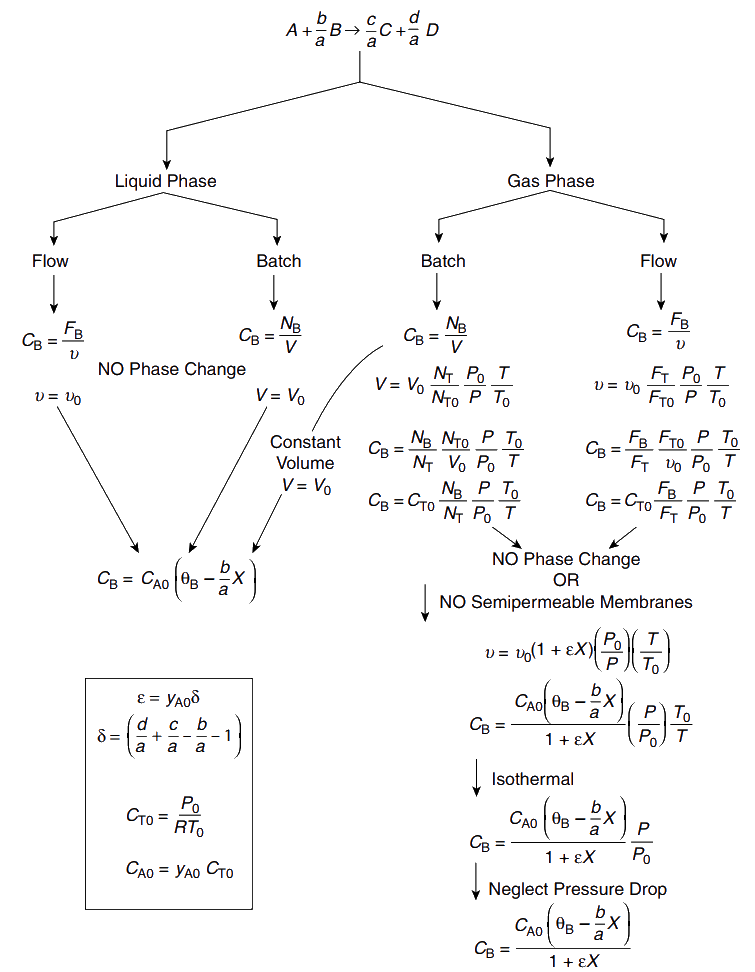
Stoichiometry
If the rate law depends on more than ne species, we must relate concentrations of different species to each other.
Stoichiometric table
Represents stoichiometric relationships between reacting molecules for a single reaction
How many molecules of one species will be formed during a chemical reaction given a number of molecules of another species disappear.
Let us consider general reaction
aA+bBcC+dD
relative rates
a−rA=b−rB=crC=d−rD
Basis of calculation: Species A
A+abBacC+adD
Batch systems
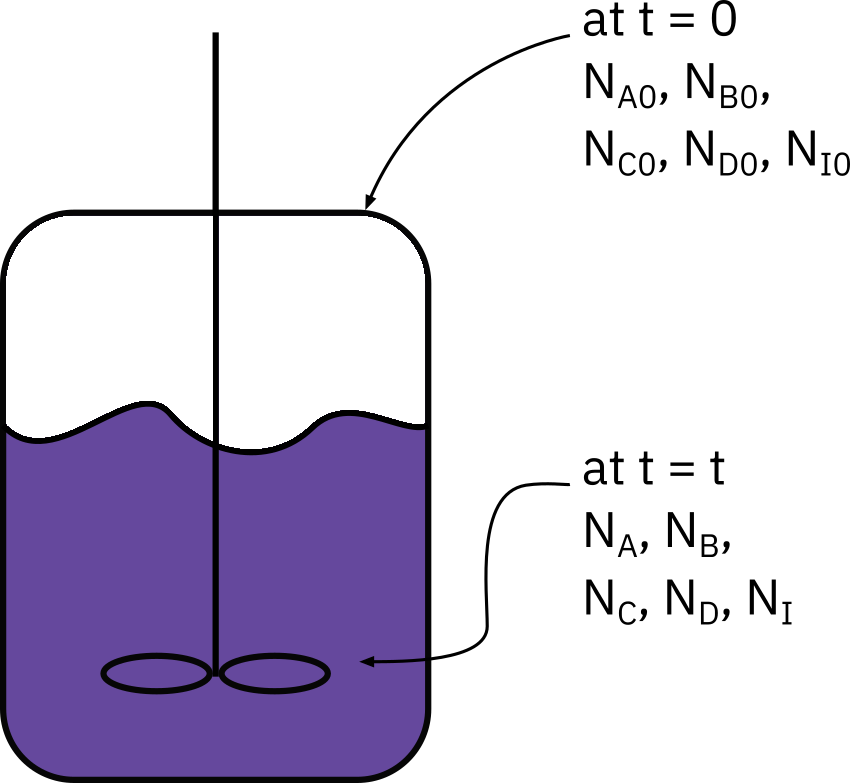
Mole balance
dtdNA=rAV
NA0 : No. of moles of A initially present
X : Conversion at time t
NA0X : No. of moles of A consumed at time t
NA : No. of moles of A in system at time t
NA=NA0−NA0X=NA0(1−X)
Stoichiometric table: batch systems
Moles of B reacted
moles of B reacted=mol A reactedmol B reacted×mol A reacted=abNA0X
Species Initially (mol) Change (mol) Remaining (mol) A NA0 −NA0X NA=NA0−NA0X B NB0 −(b/a)NA0X NB=NB0−(b/a)NA0X C NC0 (c/a)NA0X NC=NC0+(c/a)NA0X D ND0 (d/a)NA0X ND=ND0+(d/a)NA0X I NI0 0 NI=NI0 Total NT0 NT=NT0+δNA0X
δ=+ad+ac−ab−1
Change in total number of moles per mole A reacted
Equations for the concentration
CA=VNA=VNA0(1−X)
CB=VNB=VNB0−(b/a)NA0X
CC=VNC=VNC0+(c/a)NA0X
CD=VND=VND0+(d/a)NA0X
For a constant volume batch reactor, V=V0.
Let Θi=Ni0/NA0=Ci0/CA0.
- CA=CA0(1−X)
- CB=CA0(ΘB−(b/a)X)
- CC=CA0(ΘC+(c/a)X)
- CD=CA0(ΘD+(d/a)X)
Θi=Moles of species A initiallyMoles of species ’i’ initially
Equimolar: ΘB=1
Stoichiometric: ΘB=b/a
Rate expression
−rA=kCACB
−rA=kCA02(1−X)(ϕB−(b/a)X)
For CA0=CB0, and b/a=1
−rA=kCA02(1−X)2
Mole balance
dtdCA=−kCA02(1−X)2
as CA=CA0(1−X); dCA=−CA0dX
The mole balance can then be written as
(1−X)2dX=kCA0dt
In class exercise: equilibrium conversion in batch reactor
Consider the following elementary reaction with KC = 20 dm3/mol and CA0 = 0.2 mol/dm3. Find Xe for a batch reactor.
Reaction: 2AB
Rate law: −rA=kA[CA2−KCCB]
Flow systems
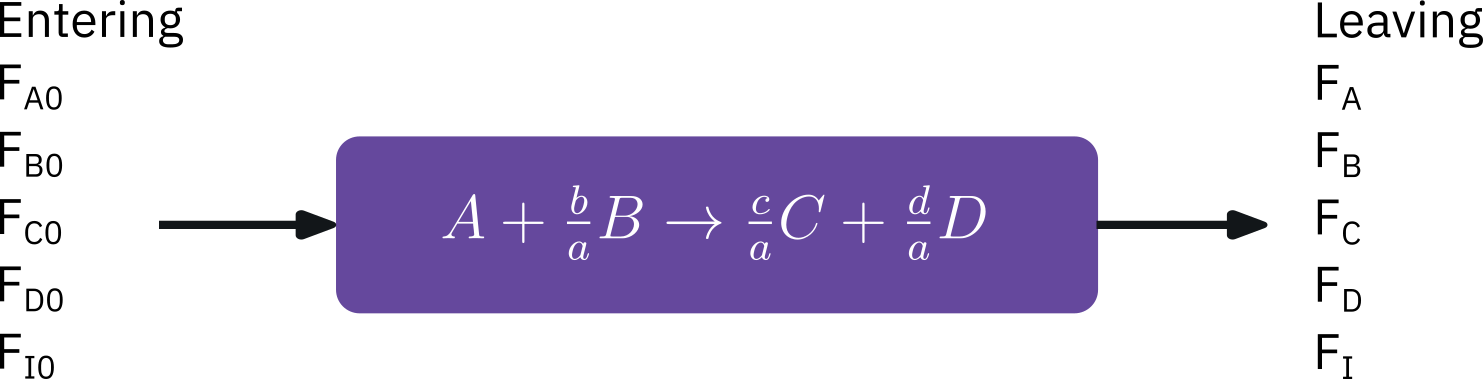
Form of stoichiometric table is virtually identical to batch systems
Replace Nj0 by Fj0
Replace Nj by Fj
Stoichiometric table: flow systems
| Species | Feed rate to reactor (mol/time) | Change within reactor (mol/time) | Effluent rate from reactor (mol/time) |
|---|---|---|---|
| A | FA0 | −FA0X | FA=FA0−FA0X |
| B | FB0 | −(b/a)FA0X | FB=FB0−(b/a)FA0X |
| C | FC0 | (c/a)FA0X | FC=FC0+(c/a)FA0X |
| D | FD0 | (d/a)FA0X | FD=FD0+(d/a)FA0X |
| I | FI0 | 0 | FI=FI0 |
| Total | FT0 | FT=FT0+δFA0X |
δ=+ad+ac−ab−1
Θi0=Fi0/FA0=Ci0/CA0.
CB=CA0(ΘB−(b/a)X)
For liquid phase systems (υ=υ0)
Gas phase reactions
Volumetric flow rate changes during the course of reaction
Changes in total number of moles
Changes in pressure and temperature
Variable flow rate
Gas phase reactions that do not have equal number of product and reactant moles
e.g. NX2+3HX22NHX3
- 4 mole reactant give 2 mole products
Stoichiometric tables
No assumptions made reagarding volume
The tables are exactly same for constant volume (constant density) and variable volume (variable density) systems
⇒ Only for concentration expressed in terms of conversion density/ volume comes into play.
Flow reactor with variable volumetric flow rate
We will use relationships for total concentration
CT=υFT...volumetric flow ratetotal molar flow rate
For gases
CT=zRTPz:compressibility factor (=1 for ideal gas)
At the entrance of the reactor
CT0=z0RT0P0
Assuming negligible change in z (z=z0)
υ=υ0(FT0FT)(PP0)(T0T)
Flow reactor with variable volumetric flow rate
We can now express Cj(=Fj/υ) in terms of F, P, and T
Cj=CT0(FTFj)(P0P)(TT0)
FT=∑j=1nFj=FA+FB+FC+FD+FI
Fj is found by solving mole balance equations
Concentration in terms of conversion: FT=FT0+FA0δX
Dividing by FT0 and defining ϵ=yA0δ, where yA0 is the mole fraction of A at inlet
FT0FT=1+ϵX
ϵ=total moles fedchange in total no. of moles for complete conversion
Flow reactor with variable volumetric flow rate
Equation for volumetric flow rate υ=υ0(1+ϵX)(PP0)(T0T)
Molar flow rate Fj=FA0(Θj+νjX)
Concentration (Cj=Fj/υ)
Cj=υ0(1+ϵX)(PP0)(T0T)FA0(Θj+νjX)
- Stoichiometric coefficient (νj)
A+ab Bac C+ad D
-ve for reactant
νA=−1; νB=−b/a
+ve for products
νC=c/a; νD=−d/a
Cj=(1+ϵX)CA0(Θj+νjX)(P0P)(TT0)
In class exercise: equilibrium conversion in flow reactor
Consider the following elementary reaction with KC = 20 dm3/mol and CA0 = 0.2 mol/dm3. Find Xe for a flow reactor.
Reaction: 2AB
Rate law: −rA=kA[CA2−KCCB]
In class exercise: saponification reaction
The saponification for the formation of soap is:
3NaOH+(CX17HX35COO)X3CX3HX53CX17HX35COONa+CX3HX5(OH)X3
Letting X represent the conversion of NaOH set up a stoichiometric table expressing the concentration of each species in terms of the initial concentration of NaOH and the conversion of X.
In class exercise: determining Cj=hj(X)
A mixture Of 28% SOX2 and 72% air is charged to a flow reactor in which SOX2 is oxidized.
2SOX2+OX22SOX3
First, set up a stoichiometric table using only the symbols (i.e., Θi,Fi).
Next, prepare a second table evaluating the species concentrations as a function of conversion for the case when the total pressure is 1485 kPa (14.7 atm) and the temperature is constant at 227 °C.
Evaluate the parameters and make a plot of each of the concentrations SOX2, SOX3, NX2 as a function of conversion
In class exercise: liquid phase first order reaction
Orthonitroanaline (an important intermediate in dyes—called fast orange) is formed from the reaction of orthonitrochlorobenzene (ONCB) and aqueous ammonia. The liquid-phase reaction is first order in both ONCB and ammonia with k=0.0017 m3/kmol⋅min at 188 ∘C with E=11273 cal/mol. The initial entering concentrations of ONCB and ammonia are 1.8 kmol/m3 and 6.6 kmol/m3, respectively.
CX6HX4ClNOX2+2NHX3CX6HX6NX2OX2+NHX4Cl
Set up a stoichiometric table for this reaction for a flow system.
Write the rate law for the rate of disappearance of ONCB in terms of concentration.
Explain how parts (a) and (b) would be different for a batch system.
Write −rA solely as a function of conversion.
What is the initial rate of reaction (X = 0) at 188 ∘C, at 25 ∘C, and at 288 ∘C?
What is the rate of reaction when X = 0.90 at 188 ∘C, at 25 ∘C, and at 288 ∘C?
What would be the corresponding CSTR reactor volume at 25 ∘C to achieve 90% conversion and at 288 ∘C for a feed rate of 2 dm3/min
Summary
Fogler, H. Scott. 2016. Elements of Chemical Reaction Engineering. Fifth edition
This component is an instance of the CodeMirror interactive text editor. The editor has been configured so that the Tab key controls the indentation of code. To move focus away from the editor, press the Escape key, and then press the Tab key directly after it. Escape and then Shift-Tab can also be used to move focus backwards.

Chemical Reaction Engineering